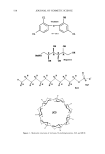
550 JOURNAL OF COSMETIC SCIENCE (18) M.M. de Villiers, W. Liebenberg, S. F. Malan, and J.J. Gerber, The dissolution and complexing properties of ibuprofen and ketoprofen when mixed with N-methylglucamine, Drug Dev. Ind. Pharm., 25,967-972 (1999). (19) A. A1-Maaieh and D. R. Flanagan, Salt effects on caffeine solubility, distribution, and self-association, J. Pharm. Sci., 91, 1000-1008 (2002). (20) K. R. Morris, M. G. Fakes, A. B. Thakur, A. W. Newman, A. K. Singh, J. J. Venit, C. J. Spagnuolo, and A. T. M. Serajuddin, An integrated approach to the selection of optimal salt form for a new drug candidate, Int. J. Pharm., 105, 209-217 (1994).
Cosmet. Sci., 54, 551-558 (November/December 2003) Comparison of antioxidant activity of extract from roots of licorice (Glycyrrhiza g/abra L.) to commercial antioxidants in 2% hydroquinone cream KATAYOUN MORTEZA-SEMNANI, MADJID SAEEDI, and BITA SHAHNAVAZ, Department of Medicinal Chemistry (K. M. -S., B.S.) and Department of Pharmaceutics (M. S.), Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran. Accepted for publication May 20, 2003. Synopsis Powdered dry roots of licorice (Glycyrrhiza glabra L.) were extracted with methanol. Licorice extract was tested for antioxidative activity in comparison with antioxidants (sodium metabisulfite and BHT) at 0.1%, 0.5%, 1.0%, and 2.0% w/w in 2% w/w hydroquinone cream. The systems were incubated in a dark room at 25 ø +_ 0.5øC and 45 ø _+ 0.5øC for three months. The physical stability and the percentages of hydro- quinone remaining after two weeks and one, two, and three months were determined by UV spectropho- tomerer at 294 nm according to official standard procedures. The experiment revealed that oxidation degradation of hydroquinone was accelerated by heat even with the existence of antioxidants. The higher percentages of remaining hydroquinone were observed for higher antioxidant concentration but showed lower physical stability in the formulation in the presence of commercial antioxidants, especially in the cases of 1.0% and 2.0% BHT. In the third month, at 25 ø _+ 0.5øC and 45 ø +_ 0.5øC, the extract demonstrated more antioxidant activity from two other commercial antioxidants at all concentrations, with about 43-53% and 34-46%, respectively, more hydroquinone remaining than in the control system (p 0.001). In the third month, the preparation containing 0.1%, 0.5%, 1.0%, and 2.0% extract gave good physical formu- lation stability with about 72%, 76%, 78%, and 81% hydroquinone remaining at 25 ø + 0.5øC and 51%, 55%, 60%, and 63% hydroquinone remaining at 45 ø + 0.5øC, respectively. This suggested the possibility of using a licorice extract at 0.5% and 1.0% as an effective natural antioxidant for substances that are oxidation-susceptible. INTRODUCTION One of the most important characteristics of many cosmetic products is stability. Hy- droquinone, a hypopigmenting agent employed percutaneously to lighten localized areas of hyperpigmented skin such as blemishes, lentigo, melasma, chloasma, and freckles, is known for its high oxidative reactivity. It is one of the chemicals that are difficult to Address all correspondence to Katayoun Morteza-Semnani. 551
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)