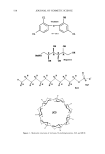
544 JOURNAL OF COSMETIC SCIENCE For the triclosan cyclodextrin complex, water should be the preferred solvent for com- plexation because the triclosan molecule that complexes with the cavity of the cyclo- dextrin is nonpolar and prefers the nonpolar environment of the cavity rather than the polar aqueous environment. As a result, water provides a driving force for complexation in addition to dissolving or dispersing the cyclodextrin and guest. Because of the high solubility of •3CD and HP•3CD, the complexes are also very soluble. In Table I the maximum solubilities obtained by cyclodextrin complexation with triclosan are listed. There is not a significant difference between the solubility obtained with •3CD and HP•3CD in both water and pH 7.4 buffer. Contrary to earlier reports in this study, the less soluble •CD was not an effective solubilizer of triclosan (14). SOLUBILIZATION WITH SLS As shown in Figures 3 and 4, SLS was an even more effective solubilizing agent than •3CD and HP•3CD for triclosan. Solubilization of triclosan by this anionic surfactant (Figure 1) occurs through the formation of micelies because when present in a sufficient concentration (above the critical micelie concentration), SLS forms miceliar aggregates with hydrophobic, organic tails in the center and anionic polar groups on the outside. These micelies trap in or between their hydrophobic cores the lipophilic triclosan molecules that enhance the solubility of triclosan by several orders of magnitude (3000- to 6000-fold, Table I). Within the experimental parameters used in this study, the increase in the solubility of triclosan achieved with SLS did not reach a plateau (Figures 3 and 4). This suggests that a further increase in the concentration of SLS could increase the solubility of triclosan even more. However, because of excessive foaming, it was difficult to analyze highly concentrated SLS solutions. SOLUBILIZATION BY AMINO ALCOHOLS AND AMINO ACIDS Based on previous reports on the solubilization of drug molecules with amino alcohols (17,18), in this study the effect of ethanolamine, diethanolamine, triethanolamine, D(+)glucosamine, and N-methylglucamine on the solubility of triclosan was investi- gated. Figures 5 and 6 are solubility profiles for triclosan when combined with these amino alcohols. D(+)glucosamine was discarded early during preliminary tests because of its poor solubilizing performance. The order in which these compounds increased the solubility of triclosan (Table I) was N-methylglucamine ethanolamine triethanol- amine -- diethanolamine, both in water and solutions buffered at pH 7.4. In both these media, the solubilization power of N-methylglucamine significantly surpassed that of the other amines, •3CD and HPI3CD, and SLS. N-methylglucamine is a derivative of sorbitol in which the hydroxyl group in position 1 is replaced by a methylamino group (Figure 1). This compound is most often used in conjunction with iodinated organic compounds as a contrast medium. The increased solubility of triclosan achieved by the amino alcohols may be attributed to the in situ formation of either a salt or a complex (17,18). It is assumed that the formation of these association compounds occurs between the electronegative nitrogen of the amines and the enolic hydrogen of triclosan. This complex is similar to other complexes formed between the electron-donating oxygen of polyethylene glycols and the acidic hydrogen
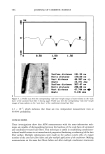
INCREASED AQUEOUS SOLUBILITY OF TRICLOSAN 545 14 12 10 -•- Meglumine -e- Ethanolamine -e- Diethanolamine + Triethanolamine 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Concentration (M) Figure 5. Solubility of triclosan (mg/m1-1) at increasing concentrations of several amino alcohols in water. The data points and error bars represent the mean and standard deviations of two replicates. 2O 16 A Meglumine -e- Ethanolamine -e- Diethanolamine T • "r T 'r I ! 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Concentration (M) Figure 6. Solubility of triclosan (mg/m1-1) at increasing concentrations of several amino alcohols in pH 7.4 phosphate buffer. The data points and error bars represent the mean and standard deviations of two replicates. of drugs such as phenobarbital (17). Such a triclosan/amino alcohol complex will form hydrogen bonds with water molecules through the hydrophilic hydroxyl groups of the alcohol moiety to increase the water solubility. In this study, it was not possible to
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)