558 JOURNAL OF COSMETIC SCIENCE licorice extract is more effective than sodium metabisulfite and BHT, and may be used as a substitute for commercial antioxidants in oxidation-sensitive formulations. REFERENCES (1) A. Manosroi, M. Abe, and J. Manosroi, Comparison of antioxidant activity of extract from seeds of white pepper (Piper nigrum, Linn.) to commercial antioxidants in 2% hydroquinone cream, J. Cosmet. Sci., 50, 221-229 (1999). (2) R. Gr Harry's Cosmeticology (Chemical Publishing Co., New York, 1996). (3) D. Mantle, F. Eddeb, and A. T. Pickering, Comparison of relative antioxidant activities of British medicinal plant species in vitro, J. EthnopharmacoL, 72, 47-51 (2000). (4) T.J. Vanderjagt, R. Ghattas, D.J. Vanderjagt, M. Crossey, and R. H. Glew, Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico, Li• Sci., 70, 1035-1040 (2002). (5) C. F. Duffy and R. F. Power, Antioxidant and antimicrobial properties of some Chinese plants, Int. J Antimicrob. Agents, 17, 527-529 (2001). (6) J. Vaya, P. A. Belinky, and M. Aviram, Antioxidant constituents from licorice roots: Isolation, struc- ture elucidation, and antioxidative capacity toward LDL oxidation, Free Radical Biol. Med., 23, 302- 313 (1997). (7) D. Mantle, J. G. Anderton, G. Falkous, M. Barnes, P. Jones, and E. K. Perry, Comparison of methods for determination of total antioxidant status application to analysis of medical plant essential oils, Comparative Biochem. Physiol. B, 121, 385-391 (1998). (8) E. H. Mansour and A. H. Khalil, Evaluation of antioxidant activity of some plant extracts and their application to ground beef patties, Food Chem., 69, 135-141 (2000). (9) J. A. Cook, D. J. Vanderjagt, A. Dasgupta, G. Mounkaila, R. S. Glew, W. Blackwell, and R. H. Glew, Use of the Trolox assay to estimate the antioxidant content of seventeen edible wild plants of Niger, Life Sci., 63, 105-110 (1998). (10) J. A. Vinson and Y. A. Dabbagh, Tea phenols: Antioxidant effectiveness of tea, tea components, tea fractions and their binding with lipoproteins, Nutr. Res., 18, 1067-1075 (1998). (11) P.A. Belinky, M. Aviram, B. Fuhrman, M. RosenNat, and J. Vaya, The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation, Atherosclerosis, 137, 49-61 (1998). (12) H. Hayashi, N. Hiraoka, Y. Ikeshiro, and H. Yamamoto, Organ specific localization of flavonoids in Glycyrrhiza glabra L., Plant Sd., 116, 233-238 (1996). (13) K. Okada, Y. Tamura, M. Yamamoto, Y. Inoue, R. Takagaki, K. Takahashi, S. Demizu, K. Kajiyama, Y. Hiragy, and T. Kinoshita, Identification of antimicrobial and antioxidant constituents from licorice of Russian and Xinjiang origin, Chem. Pharm. Bull., 37, 2528-2530 (1989). (14) B. Fuhrman, S. Buch, J. Vaya, P. A. Belinky, R. Coleman, T. Hayek, and M. Aviram, Licorice extract and its major polyphenol glabridin protect low-density lipoprotein against lipid peroxidation: In vitro and ex-vivo studies in humans and in atherosclerotic apolipoprotein E-deficient mice, Am. J Clin. Nutr., 66, 267-275 (1997). (15) S. Bemizu, K. Kajiyama, K. Takahashi, Y. Hiraga, S. Yamamoto, Y. Tamura, K. Okada, and T. Kinoshita, Antioxidant and antimicrobial constituents of licorice isolation and structure elucidation of a new benzofuran derivative, Chem. Pharm. Bull., 36, 3474-3479 (1988). (16) M. H. Gordon and J. An, Antioxidant activity of flavonoids isolated from licorice, 43, 1784 (1995). (17) F. S. D'Amelio, Botanicals: A Phytocosmetic Desk Refirence (CRC Press, Boca Raton, FL, 1999). (18) A. Y. Leung and S. Foster, Encyclopedia of Common Natural Ingredients Used in Food, Drags and Cosmetics (Wiley-Interscience, New York, 1996). (19) United State• Pharmacopoeia (USP), XXIV (2000).
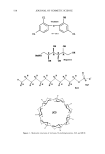
j. Cosmet. Sci., 54, 559-568 (November/December 2003) Solubilization of sodium cocoyl isethionate JAMES ZIMING SUN, JAMES W. PARR, and MICHAEL C. E. ERICKSON, Advanced Research Laboratories, 151 Kalmus Dr. Suite H-3, Costa Mesa, CA 92626. Accepted for publication July 15, 2003. Presented at the Annual Scientific Seminar of the Society of Cosmetic Chemists, Washington, DC, May 9, 2003. Synopsis Sodium cocoyl isethionate (SCI) has been a predominant ingredient in syndet bar formulation for more than thirty years. Although cost effective and well recognized for good skin compatibility, SCI is not regularly found in liquid detergent systems due to its limited solubility in water. This study focuses on the under- standing of enthalpy of solubilization, equilibrium of solubilization, and the structures and properties of sodium cocoyl isethionate and various surfactants. The purpose of this exercise is to help the formulator to find appropriate surfactant systems to keep sodium cocoyl isethionate in aqueous solution. The solubility of SCI in water is unfavorable in terms of enthalpy of solvation. When setting up equilibrium of solubilization, there are three possible phases, and three methods have been developed to prevent SCI from recrystallizing in aqueous solutions. The first focuses on tying CI ions within micelies made of secondary surfactants. The second focuses on the exchange of sodium ions with ammonium ions (and/or triethanola- monium). The third centers on emulsification of SCI and the subsequent change of micelies into emulsified oil drops. A combination of two or three of these methods will enable the formulator to use SCI as the primary surfactant in liquid detersive systems. INTRODUCTION Owing to its excellent skin compatibility, mildness, and emollient properties, sodium cocoyl isethionate (SCI) has been used extensively in syndet bars (1-6). SCI has become cost efficient because of its broad application in personal care. Chemists try to incor- porate SCI 'within liquid detersive systems in order to take advantage of both its excellent performance characteristics and its cost efficiency. However, SCI is not widely used in liquid cleansing systems because of its limited solubility in water. Some proprietary technologies have been developed to increase the aqueous solubility of SCI (7-9). Zwitterionic detergents (betaines) were discovered to increase the solubility of SCI in liquid detersive compositions (7). In such systems, SCI, betaine(s), and other anionic surfactants (other than SCI) are the three major surfactants. Alkylamphoacetates are another category of surfactants disclosed to increase the solubility of SCI (8). N- methyl cocoyl tautate and laureth sulfate are also used as major surfactants in these proprietary technologies. Nonionic sugar surfactants selected from the group consisting 559
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)