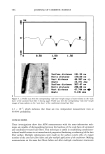
546 JOURNAL OF COSMETIC SCIENCE isolate these association complexes from solution. This provides some indication of the instability of these complexes. Although slight increases in the pH of the media resulted from an increase in amino alcohol concentration, solubilization of triclosan by this increase in pH was not signifi- cant because even higher concentrations of triclosan were achieved in solutions buffered at pH 7.4 (Table I). At a concentration of 1.0 M N-methylglucamine (Table I), the solubility of triclosan in the phosphate-buffered solution was =50% higher than in water. This phenomenon could be attributed to an increase in the solvent activity caused by the buffer salts in combination with N-methylglucamine (salting-in), leading to an increase in the solubility of the complexes (19). The same media-dependent increase in solubility was not observed with the other amino alcohols but was observed for L- arginine. Figures 7 and 8 represent solubility profiles of triclosan in combination with two highly water-soluble amino acids and two water-soluble preservatives. The two preservatives, sodium benzoate and sodium methyl 4-hydroxybenzoate, increased the solubility of triclosan, but this increase was not as significant as that obtained with N- methylglucamine, [3CD, HP[3CD, and SLS. However, the combination of these preser- vatives with triclosan could prove useful for the formulation of products where preser- vative combinations might reduce the chance of bacterial resistance. The acidic amino acid glycine (Figure 7) did not increase the solubility of triclosan, but the strongly alkaline L-arginine did increase the solubility of triclosan in aqueous solutions. The mechanism whereby it increases the solubility of triclosan is probably similar to that of N-methylglucamine because of structural similarities, especially the amine groups (10). 14 12 10 •, Glycine -e- L-Arginine - Na-Benzoate + Na-Methylparaben 4. 4, .I. .I. 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Concentration (M) Figure 7. Solubility of triclosan (mg/ml -]) at increasing concentrations of two amino acids and other preservatives in water. The data points and error bars represent the mean and standard deviations of two replicates.
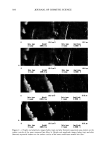
INCREASED AQUEOUS SOLUBILITY OF TRICLOSAN 547 2O -•- Glycine 16 -e- L-Arginine ..• ß Na-Benzoate = 8 0.0 0.2 0.4 0.6 0.8 1.0 1.2 Concentration (M) Figure 8. Solubility of triclosan (mg/ml -•) at increasing concentrations of two amino acids and other preservatives in pH 7.4 phosphate buffer. The data points and error bars represent the mean and standard deviations of two replicates. There was no significant difference in the solubility profiles (Figure 7 and 8) of triclosan combined with N-methylglucamine or L-arginine in both water and the buffer. Maxi- mum solubilities achieved at corresponding concentrations of the solubilizers were also not significantly different (Table I). EFFECT OF SOLUBILIZERS ON THE ANTIMICROBIAL ACTIVITY OF TRICLOSAN Triclosan possesses bacteriostatic activity at low concentrations when tested against most gram-negative as well as gram-positive bacteria by the agar incorporation method, a notable exception being Pseudomonas (1). Since the solubilizers increased the solubility of triclosan in water, it is important to know if these solubilizers influenced the antimi- crobiological activity of triclosan. Therefore, the antimicrobial activity of the five solu- bilizers that improved the solubility of triclosan in water the most, N-methylglucamine, L-arginine, ethanolamine, SLS, and [3CD, were tested alone and in combination with triclosan against Escherichia co/i, Pseudomonas aeruginosa, Staphylococcus aereus, Aspergillus niger, and Candida albicans. The size of growth inhibition zones listed in Table II show that the solubilizer with the best bacteriostatic activity was SLS against A. niger. However, this compound did not inhibit the growth of E. co/i or P. aeruginosa. Both N-methylglucamine and ethanolamine significantly inhibited the growth of all the organisms tested, while [3CD showed no zone inhibition at all. Triclosan inhibited the growth of all the organisms tested except P. aeruginosa. Statistically there were no significant differences between the growth inhibition zones
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)