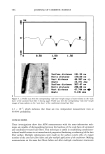
556 JOURNAL OF COSMETIC SCIENCE ing level of hydroquinone than those incubated at 45øC (p 0.05). This indicated the effects of temperature in accelerating the oxidative degradation of hydroquinone (Figure 1). Figures 2-5 compare the average percentages of hydroquinone remaining in 2% w/w hydroquinone cream containing licorice extract and the commercial antioxidants kept at 25øC and 45øC for three months. Both water-soluble antioxidant (sodium metabisulfite) and oil-soluble antioxidant (BHT), as well as the extract at all concentrations, showed more hydroquinone remaining than in the control system after incubation at 25øC and 45øC for three months, with the exception of 0. ! % SM and 0.1% BHT systems at 45øC (p 0.001). The difference in antioxidant activity between the systems of extract and SM or BHT at all concentrations was not significant at 25øC after two weeks (p 0.05). This difference was not observed between the systems of extract and BHT at all concentrations at 25øC after one and two months (p 0.05). The licorice extract at all concentrations showed more hydroquinone remaining than in the systems of SM and BHT at 25øC and 45øC after three months (p 0.05). The comparison of antioxidant activity of licorice extract systems showed that there was no significant difference between 1.0% and 2.0% extract systems at 25øC and 45øC during a three-month period. Both water-soluble and oil-soluble antioxidants and licorice extract in our study showed some protection from oxidative degradation in hydroquinone (but not 100% protection) during a three-month period. The extract systems were more effective than other com- A 9O 9O 8O 8O i 70 i 70 60 60 50 50 i 30 30 20 20 10 10 0 B i i i i 0 2 4 6 8 •0 •2 0 2 4 6 8 Time (week) Time (Week) i i 10 12 •CB 1% I '"'-•CB 1% J• SM1% .(
•Ext. BHT1% J• MS 1% .(
•Ext. BHT1% Figure 4. Formulation stability study of 2% w/w hydroquinone cream containing 1.0% extract and commerical antioxidants incubated at 25 ø _+ 0.5øC (A) and 45 ø _+ 0.5øC (B) for three months.
ANTIOXIDANT ACTIVITY OF LICORICE EXTRACT 557 lOO 90 80 70 60 50 40 30 20 lO o o A i i i i i 4 6 8 10 12 Time (Week) B lOO 9o 8o 70 50 30 20 10 0 2 4 6 8 10 12 Time (week) C CB .--•Ext. 2% C CB .--•Ext. 2% .& SM2% ,( BHT2% .& SM2% ,( BHT2% Figure 5. Formulation stability study of 2% w/w hydroquinone cream containing 2.0% extract and commercial antioxidants incubated at 25 ø _+ 0.5øC (A) and 45 ø _+ 0.5øC (B) for three months. mercial antioxidants. According to our procedure, by incorporating hydroquinone after the cream was formed, and together with the solubility property of hydroquinone that is freely soluble in propylene glycol and slightly soluble in oil and water, we had expected that hydroquinone had been absorbed in both the oil and the water phases of the formulation. It is also evident that hydroquinone can be incorporated in the for- mulation in different ways. Besides our procedure, hydroquinone was used in the oil phase with laevo-ascorbic acid as antioxidant, and in the water phase with sodium metabisulfite and ascorbic and citric acids as antioxidants (1). This meant that the types of antioxidants selected for use in the formulation depended on the method of the incorporation of hydroquinone into the system. In our hydroquinone systems containing extract, the licorice acted as both water- and oil-soluble antioxidant agents and dem- onstrated significant protection from oxidative degradation in hydroquinone for three months, in comparison with sodium metabisulfite and BHT that were water-soluble and oil-soluble, respectively. CONCLUSION The licorice extract at 0.5% and 1.0% can be used as a double-action (both water- and oil-soluble) antioxidant, having 76% and 78% (at 25øC) and 55% and 60% (at 45øC) of hydroquinone remaining, respectively, after three months. These results indicate that
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)