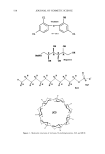
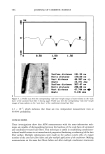
572 JOURNAL OF COSMETIC SCIENCE .•Onm • 50Ohm Figure 1. TEM images of biphasic polymer latexes: (a) PBMA. (b) NAS300. (c) NAS! 100. (d) NAS2000. DLS measurement, even though the effect of the PEG chains shows a similar trend, the latex sizes are somewhat larger than those determined by TEM measurement. This result is related to the effect of hydrodynamic volume. In the aqueous dispersion, the PEG- ylated latexes have a larger hydrodynamic volume, compared with non-treated latexes. In order to examine the effect of a cationic monomer on the latex formation, the emulsion polymerization was carried out in the presence of 5 wt% of METAC. The latex size was remarkably reduced by incorporating the cationic group into the polymer backbone. This reveals that the cationic group makes a big contribution in improving the electrostatic stability of the latex particles (11). The composition and size charac- teristics measured are summarized in Table I. FILM PROPERTIES OF BIPHASIC LATEXES In order to examine the film property, the biphasic latexes were cast in a Teflon molder. The film prepared with the latexes containing 2000 g ß tool • of PEG was very sticky due to the high molecular weight, resulting in a very soft continuous film phase. Therefore, considering basically the film properties, it was established that the molecular weight of PEG should be located between 300 and 1100 g ß tool •. In this consider- ation, the molecular weight of PEG was selected at 300 g ß mol -•. Figure 2 shows the DSC thermograms of the biphasic latex films. PBMA and NAS300 films show only one transition temperature, at around 26øC. On the other hand, NAS300-M5 shows two transition temperatures at -57.5øC and 25.5øC, respectively. The results indicate that
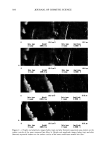
POLYMER LATEXES ON THE HAIR SURFACE 573 E 57.5øC • 25.5oc 26.5øC 26øC -------_ t ß ß i ß -100 -50 0 50 100 Temperature (øC) Figure 2. DSC thermograms of biphasic polymer latex films: (a) PBMA. (b) NAS300. (c) NAS300-M5. the phase separation between PBMA and PEG was facilitated by the introduction of the cationic group into the polymer backbone. It has been known that in the polymer latex system, a high transition temperature contributes to the formation of films on the hair surface, while a low transition temperature exhibits a good deposition profile (7). It is then possible to say that the biphasic latex system having two transition temperatures can achieve the two functions simultaneously. Figure 3 shows the mechanical property of biphasic polymer latex films. In the stress- strain curves, PBMA latex film shows a typical rigid polymer pattern. However, by adding PEG into the polymer composition, the tensile elongation increases on the other hand, the tensile strength decreases steeply. That is, the property of PBMA latex film changes from brittleness to flexibility with the aid of soft PEG moiety. The incorpo- ration of cationic groups slightly lowers tensile elongation, but the stiff property of PBMA latex film can be modified satisfactorily. In the analysis of mechanical property, it is evident that the biphasic latex film has viscoelasticity. APPLICABILITY TO HAIR COSMETICS To evaluate the ability to form a film on the surface of human hair, the biphasic latexes were deposited on the hair and the texture was examined with SEM. Figure 4 shows SEM images of latex films on the hair surface. It is clear that that the biphasic latexes readily covered the hair, resulting in a fine membrane on the hair surface. The coating ability was dependent on the functional group in the latexes. Especially, the cationic group played a crucial role in determining the degree of deposition of latexes on the hair surface. In the absence of the cationic group (NAS300), the deposition of biphasic latexes progressed partially (Figure 4b). However, in the presence of the cationic group (NAS300-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)