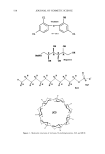
560 JOURNAL OF COSMETIC SCIENCE of alkyl glucose esters, aldobionamides, gluconamides, glyceramides, glyceroglycolipids, polyhydroxy fatty acid amides, and alkyl polyglycosides can also increase the solubility of SCI (9). Selected sugar surfactants, SCI, and free fatty acid are the three major ingredients in most proprietary compositions. These proprietary technologies do not explain why some ingredients dissolve SCI in terms of fundamental physical principles. This article will try to bridge the gap in this understanding by focusing on the enthalpy of solubilization, the equilibrium of solu- bilization, and the structures and properties of SCI and various surfactants. MATERIALS The trade names of materials used in this study are as follows: sodium cocoyl isethionate (85% active): Hostapon SCI 85 © (Clariant GmbH) and Jordapon CI Prill © (BASF) ammonium cocoyl isethionate (25% active): Jordapon ACI-30 G © (BASF) emulsifying wax NF: Polawax © (Croda) disodium laureth sulfosuccinate: Mackanate EL © (Mcintyre) polysorbate 20 and polysorbate 80:Tween-20 © and Tween-80 © (Uniqema). There are many other common surfactants from the Mcintyre Group Ltd, Chemron Corporation, and the Stepan Company. PRINCIPLES Enthalpy of solvation will be used to understand why ammonium cocoyl isethionate (ACI) is more soluble than SCI. Hess's law states that the enthalpy of a reaction is the same whether the reaction takes place in one or several steps (conservation of energy). Born and Haber (10) applied Hess's law to the enthalpy of solubilization of ionic compounds in water (Figure 1). The overall enthalpy of the solubilization process is the sum of two terms: the enthalpy of ion dissociation from the lattice matrix (lattice energy) and the enthalpy of introducing the dissociated ions into the solvent (solvation energy). AHsolubilization = U +AHsolvatio n. Size and other characteristics of ions are two main factors in determining lattice energy and solvation energy. Statistical calculation and experimental analysis show that the enthalpy of solvation is of roughly the same order of magnitude as the enthalpy of lattice energy. Thus the total change of enthalpy of solubilization can be either positive, negative, or zero, depending upon the particular compound. In cases where the enthalpy M-•X'(s) M +(•+ X'(• solubilization •-• M(H20)x+(i) + X(H20)y'(l ) Figure 1. Born-Haber cycle of solubilization of ionic compounds.
SOLUBILIZATION OF SCI 561 is negative, zero, or slightly positive, solubilization will take place. If the enthalpy change is too positive, solubilization will not occur. This is to say that ions within the lattice structure will enter solution if the interactions between solute and solvent are strong enough to overcome the lattice energy. The enthalpy of solubilization of SCI is slightly positive in terms of the Born-Haber cycle at room temperature. In another words, the lattice energy of SCI is slightly larger than the combined solvation enthalpy of sodium ion (Na +) and cocoyl isethionate ion (CI-). Sodium cocoyl isethionate has a solubility of about 0.01% by weight at 25øC in water. Alternatively, ammonium cocoyl isethionate (ACI) is very soluble in water at 25øC. A liquid surfactant in the market has 25% active ACI (by weight). However a 25% active liquid solution of ACI is more costly than an 85% active solid of SCI. Thus a substantial use of ACI within shampoos or liquid cleansing formulas would be limited to more expensive "high-end" products. SCI and ACI differ chemically only in their cationic ions, Na + and NH4 +, respectively. Here we focus on the size and characteristics of these cationic ions. Since the ammonium ion is larger in size and has a smaller solvation enthalpy than the sodium ion, -240.1 kJ mol -• for the sodium ion and -132.51 kJ mol -• for the ammonium ion (11), ACI is determined to have smaller lattice energy than SCI because the enthalpy of solubili- zation of ACI should be negative or slightly negative. Smaller lattice energy means that the energy necessary to solvate ACI should be relatively low in comparison to that of SCI. This will become an important point in the discussion of method II. Unfortunately the lattice energy of ACI and SCI, and the solvation enthalpy of CI- ion, are not available in the chemical literature for theoretical calculation. Chemists can find ways to prevent SCI from recrystallizing in aqueous solution at 25øC and below. The following discussion will focus on reaction equilibrium as a means of understanding how to make SCI more soluble in water. When SCI is dissolved in water, the equilibrium reaction is as follows: NaCI(s ) • Na+(1) + CI-o) • Na+(1) + MIC(CI-)(1 ) Le Chatlier's theorem states that if a system in equilibrium is disturbed, it acts to minimize the disturbance. Any change will make the reaction shift to either the right or left in order to reestablish equilibrium. The phase separation model (6) tells us that micelles constitute a new phase, formed in the system at and above the critical micelle concentration. The equilibrium reaction indicates that it is reasonable to assume there are three possible phases involved in the equilibrium: recrystallizing solid (SCI), mo- nometic surfactant ions (Na + and CI ), and micelles (MIC) of pure CI-. Decreasing the concentration of CI-, Na +, or MIC(CI) will shift the reaction equilibrium to the right, which leads to a reduction of solid SCI the solubility of SCI is increased. Increasing the concentration would shift the equilibrium to the left and decrease the solubility of SCI. Krafft temperature is often used to describe the temperature-dependent solubility of surfactants in water. Surfactant solubility will undergo a sharp, discontinuous increase at some characteristic temperature referred to as the Krafft temperature, T x. At tempera- tures below T x, solubility is determined by the crystal lattice energy (U) and the enthalpy change of hydration of the system AHsolvation, as stated in the Born-Haber cycle. At temperatures above Tx, the solubility of the surfactant monomer may increase to the point at which micelle formation begins and aggregate forms become thermo-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)