HAIR FIBER HYDRATION 535 (11) J. Cao, Melting study of the o• form crystallites in human hair keratin by DSC, Thermodyn. Acta, 335 (1999). (12) F.J. Wortmann, C. Springob, and G. Sendelbach, Investigations of cosmetically treated human hair by differential scanning calorimetry in water, IFFCC. Ref 12 (2000). (13) S. Paterson, N. McLachlan-Troup, R. Cordero, M. Dohanal, and S. Carman, Qualitative screening for drug abuse in hair using GC-MS, J. Anal. Toxicol. 25, 3 (2001). (14) N. Tanada, S. Kashimura, M. Kageura, and K. Hara, Practical GC/MS analysis of oxidation dye components in hair fiber as a forensic investigative procedure,J. Forensic Sci, 44, 2 (1999). (15) P.S. Bonato, Introdug•o aos m•todos cromatogr•ficos [Introduction to chromatographic methods], 6th ed. (Ed Unicamp, 1995). (16) A. Agresti, Ed., Categorical data analysis (John Wiley & Sons, New York, 1990). (17) T. C. S. Dias, A. Donolato, and A. Gomes, Hidratag•o e proteg•o do cabelo & os silicones [Hair hydration and protection & silicones], Anais do Congresso ABC (2000). (18) J. Close, The concept of sensory quality, J. Soc Cosmet Chem., 45, 95-107 (1994). (19) L. Rigano, S. Sirigu, and S. Giogilli, Sensory evaluation in cosmetic fields: An overview, Cosmet. Toiletr. Manufac. Worldwide, 247-250 (1999).
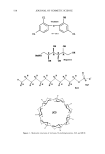
j. Cosmet. Sci., 54, 537-550 (November/December 2003) Improving the aqueous solubility of triclosan by solubilization, complexation, and in situ salt formation CHRISTINE GROVE, WILNA LIEBENBERG, JAN L. DU PREEZ, WENZHAN YANG, and MELGARDT M. DE VILLIERS, School of Pharmacy, Potche•troom University for Christian Higher Education, Potchefitroom 2520, South Africa (C.G., W.L., J.L.d.P.), and Department of Basic Pharmaceutical Sciences, School of Pharmacy, University of Louisiana at Monroe, Monroe, LA 71209 (W.Y., M.M.d.V.). Accepted for publication July 29, 2003. Synopsis Triclosan, an antimicrobial, although widely incorporated into many skin care products, toothpastes, and liquid soaps, presents formulation difficulties because it is practically insoluble in water. The objective of this study was to improve the aqueous solubility of triclosan through solubilization, complexation, and salt formation. The solubility of triclosan in distilled water and in phosphate buffers (pH 7.4) was determined at 30øC. The order of solubilizing performance of the solubilizers was: N~methylglucamine -- L-arginine sodium lauryl sulfate [3-cyclodextrin -- hydroxypropyl-[3-cyclodextrin ethanolamine sodium benzoate sodium methyl 4-hydroxybenzoate triethanolamine -- diethanolamine. These solubilizers increased the solubility of triclosan from 80- to 6000-fold. Micellar solubilization and the formation of either salts or complexes are postulated as possible mechanisms for the increase in the solubility of triclosan by the surfactant sodium lauryl sulphate, the cyclic sugar derivatives [3-cyclodextrin and 2-hydropropyl- [3-cyclodextrin, the amino acid L-arginine, and the amino sugar alcohol N-methylglucamine. Furthermore, although the bacteriostatic efficacy of triclosan was significantly increased when solubilized with N- methylglucamine, L-arginine, and ethanolamine, increased solubilization did not increase the effectiveness of triclosan for all solubilizers tested. INTRODUCTION Triclosan, also known as 5-chloro-2-(2,4-dichlorophenoxy) phenol, 2,4,4'-trichloro-2'- hydroxydiphenylether (Figure 1), is an antimicrobial that is incorporated into many skin care products, toothpastes, liquid soaps, carpets, children's toys and plastic kitchenware (1,2). It has antimicrobial activity against gram-negative as well as gram-positive bac- teria, under both in vitro and in vivo conditions (1). It is a white to off-white crystalline powder with a faint aromatic smell, a melting point of 55ø-57øC, a pKa of 7.9, and Address all correspondence to Melgardt M. de Villiers. 537
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)