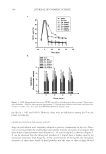
J. Cosmet. Sci., 61, 73–83 (March/April 2010) 73 In vitro/in vivo and analytical evaluation of sunless tanning formulations containing different rheology modifi ers OLGA V. DUEVA-KOGANOV, YAMINI MANDALIA, JUAN BRITO, COLLEEN ROCAFORT, STEVEN OROFINO, and GUSTAVO VAZQUEZ, Ciba Corporation (Ciba is part of BASF Group), Tarrytown, NY. Accepted for publication August 10, 2009. Synopsis In vitro data suggest that different in vivo performances are expected for two dihydroxyacetone (DHA)- containing formulations with similar concentrations of DHA and excipients but different commercially available rheology modifi ers: one with a cationic polymer-based rheology modifi er (blend) [dimethylacrylam- ide/ethyltrimonium chloride methacrylate copolymer (and) propylene glycol dicaprylate/dicaprate (and) PPG-1 trideceth-6 (and) C10-11 isoparaffi n] and the other with a polyacrylamide-based rheology modifi er (blend) [polyacrylamide (and) C13-14 isoparaffi n (and) laureth-7]. Both rheology modifi ers (blends) con- tained comparable levels of polymers and were used at 3% w/w (as supplied). Differences in color develop- ment were illustrated in vitro with respect to the yellow/red and lightness/chroma parameters, which were confi rmed in the followup in vivo studies. The test article with the cationic polymer-based rheology modifi er produced a more natural sunless tan, comparable to a desirable sun-induced tan, for all panelists, one that was more uniform and lasted longer compared with the sunless tan generated by the test article with the polyacrylamide- based rheology modifi er. A method for HPLC analysis of DHA in sunless tanning formulations was estab- lished and utilized to confi rm concentrations of DHA in test articles. INTRODUCTION A tanned appearance is considered a symbol of a healthy and active life. A sunless tan is generated by the Maillard reaction of DHA and/or erythrulose with the amino acid groups in peptides and proteins in the stratum corneum. DHA is a simple three-carbon keto- sugar obtained by fermentation of glycerin. The Maillard reaction, fi rst described in 1912 by Louis-Camille Maillard, occurs between sugars and amino acids, peptides and pro- teins, and produces dark pigments called melanoidins. Sunless tanning products contain DHA in concentrations ranging from about 1.25% to 15%. Most drugstore products range from 3% to 5%, with professional products ranging from 5% to 15%, correspond- ing to product coloration levels from light to dark. The sunless tan usually takes two to four hours to appear on the skin surface, and continues to darken for 24 to 72 hours, depending on formulation type. DHA does not damage the skin, and is considered a safe skin-coloring agent. DHA-based sunless tanning has been recommended as a safer alternative to sun exposure by the Skin Cancer Foundation, the American Academy of
JOURNAL OF COSMETIC SCIENCE 74 Dermatology Association, the Canadian Dermatology Association, and the American Medical Association. Mintel says in its recent report that users of sunless tanning prod- ucts in the U.S. are more receptive to new products compared with users of more mature personal-care categories and that a new entrant that produces signifi cantly better tanning results could make a signifi cant dent in the position of leading brands. Increasing aware- ness of the health risks associated with sun exposure motivated 39% of those surveyed to try sunless tanners. Among those consumers who have stopped using sunless tanners, 42% gave the reason that the products are too hard to apply, while 33% cited the prod- ucts’ “artifi cial” appearance (1). Different skin types may react differently with DHA due to the individual amino acid content, moisture level, skin tone, pH, and thickness. The result could be an uneven tan, one that is too dark or too light, or an orange color. It is known that various chemicals can modify or enhance the tanning reaction obtained with DHA on skin. Examples of such ingredients include amino acids (2), amino-substituted silicone compounds (3), polyacrylamide (4), amphoglycinate (amphoacetate) derivatives (5), thickeners, humectants, UV fi lters, vitamins, and emollients (6), and strong anti- oxidants (7). Certain thickeners, such as carbomer-type polyacrylates, when combined with DHA produced malodor and/or browning of the composition (4). However, the in- formation regarding the impact of rheology modifi ers on the development of sunless tan in vitro/in vivo and the analytical methods for DHA analysis in fi nished goods formula- tions is limited. Our objectives in this study were: • To evaluate and compare the ability of two DHA-containing sunless tanning formula- tions with similar excipients but different rheology modifi ers (blends), one with a cationic polymer-based rheology modifi er and the other with a polyacrylamide-based rheology modifi er, to infl uence the sunless color development in vitro and in vivo • To establish an analytical method to determine DHA concentration in sunless tan- ning formulations. MATERIALS AND METHODS TEST ARTICLES Rheology modifi ers were incorporated at 3% w/w levels (as supplied) in test formulations X and K, containing similar concentrations of DHA and the excipients: • Test article X with cationic polymer-based rheology modifi er [INCI: dimethylacryl- amide/ethyltrimonium chloride methacrylate copolymer (and) propylene glycol di- caprylate dicaprate (and) PPG-1 trideceth-6 (and) C10-11 isoparaffi n], recently introduced to the market by Ciba Corporation (part of BASF Group). • Test article K with polyacrylamide-based rheology modifi er [INCI: polyacrylamide (and) C13-14 isoparaffi n (and) laureth-7], from Seppic. The concentrations of active polymers are comparable in both commercial rheology mod- ifi ers (blends). The polyacrylamide-based rheology modifi er utilized in test article K was selected for this comparative evaluation because it was successfully used in sunless tanners demonstrating good effi cacy (4). Formulations of the test articles are presented in Table I.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)