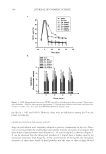
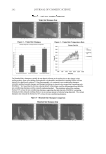
COMPARISON OF DIFFERENT PEARL POWDERS 135 In recent years, people have bred freshwater pearls as a substitute for natural pearls that occur in seawater. They have also developed water-soluble pearl (P-w) as well as ultra- micro (P-μ) and ultra-nano pearl powder (P-n) products, which have appeared in the cosmetics, health care, and medicine markets. However, there have been no scientifi c data to verify the effi cacy of these products until now. In this study, the P-w, P-μ, and P-n products were compared using moisturizing effi ciency analysis, a tyrosinase blocking test, and multiple tests for oxidation resistance. MATERIALS AND METHODS CHEMICALS Ascorbic acid (L-Vit. C), butylated hydroxyanisole (BHA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), ethylenediaminetetraacetic acid (EDTA), ferrous ammonium sulfate, ferrozine, tyrosine, and tyrosinase were purchased from Sigma Chemical Co. (St. Louis, MO). Ferric chloride, iron chloride, potassium ferricyanide, potassium hydroxide, sodium carbonate, sodium phosphate, sodium sulfate, and trichloroacetic acid were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Dimethylsulfoxid (DMSO) was obtained from Germany. One percent sodium hyaluronate (Hya) was obtained from Union Chemical Works (Tainan, Taiwan). Arbutin and seaweeds were obtained from SETALG® (France), whereas Spiraea formosana hayata powder, ginkgo powder, taraxacum powder, and Angelica dahurica powder were purchased from an herbal market in Taiwan. MATERIALS (a) The P-w and the P-μ (sieve size = 8000) were purchased from an important freshwa- ter pearl-breeding center in Zhejiang, China. (b) The P-n was purchased from Qing Dynasty Medicine King Tongrentang of Taiwan. It was examined by Elements Bio-tech Co., Ltd. with an atomic force microscope, and the results showed that the particle diameter was 117.1 ± 19.5 nm on average. (c) Spiraea formosana hayata (Spi) powders, produced by grinding, were diluted to 10 mg/ ml. In traditional Chinese medicine, Spiraea species have been used as detoxifying, analgesic, and anti-infl ammatory agents (9). These were used as a negative comparison for humidifi cation in this assay. (d) Hya, a high-molecular-weight glycosaminoglycan of the extracellular matrix of skin, also contributes to the hydration of skin and is a natural moisturizing factor (10). (e) Ginkgo powders were produced by grinding ginkgo. The superoxide-scavenging ef- fect and the antioxidant activity of the ginkgolides were adopted as a control in the antioxidant activity assays (11). (f ) Taraxacum (Tara), ground into powder, has been widely used as a folkloric medicine to treat diverse diseases and is also a naturally occurring antioxidant (12). It is used in the antioxidant activity assays as a control. (g) Arbutin, named p-hydroxyphenyl-β-D-glucopyranoside, can be extracted from plants. It is a well-known tyrosinase inhibitor and has been widely used for the whitening of skin (13).
JOURNAL OF COSMETIC SCIENCE 136 (h) Angelica dahurica (Ange-da) powders were produced by grinding Angelica dahurica. It has been reported that the ethyl acetate extract of Angelica dahurica has potent inhib- itory activity against mushroom tyrosinase. The chemical structure of the compound was identifi ed as 9-hydroxy-4-methoxypsoralen (14). (i) Seaweeds: There are a number of antioxidant and antiproliferative activities of extracts found in a variety of edible seaweeds (15). ANALYSIS OF THE EFFICACY OF MOISTURIZING A total of 16 healthy female students (20.0 ± 0.8 yr) were selected. Measurements of the hydration state of the skin were performed at 23° ± 2°C room temperature and at a relative humidity of 45 ± 5%. The TEWL was measured using an evaporimeter (Tewameter MPA5, Courage & Khazaka, Germany), whereas the hydration state of the skin was obtained using the CORNEOMETER® CM825 (Courage & Khazaka). Both TEWL and the hydration state were measured in a marked area on the front of the left hand. Ultra-micro (sieve size = 8000) pearl powders and P-w were diluted with distilled (DI) water. The arm was cleansed one hour before the test, and seven areas with a diameter of 1.5 ± 0.2 cm were defi ned on the surface of the lower arm. One test was carried out fi ve minutes before apply- ing the sample, and then a test was carried out every fi ve minutes for 30 minutes. TYROSINASE INHIBITION ASSAY Tyrosinase inhibitory activity test was performed according to the method proposed by Rout and Banerjee (16). with minor modifi cations. Briefl y, the reaction mixture contained 1 ml of 0.03% L-tyrosine, 0.9 ml of 25 mM phosphate buffer (pH = 6.8), and 1 ml of dif- ferent concentrations of samples. After ten minutes of incubation, 0.1 ml of 350 U/ml mushroom tyrosinase was added. The optical density was taken at 475 nm after 30 minutes of incubation. The percentage of inhibition of tyrosinase activity was calculated as: % inhibition 100 = − − − − × ( ( A B C D) A B where A is the absorbance of a blank solution after incubation, B is the absorbance of the blank solution before incubation, C is the absorbance of the sample solution after incuba- tion, and D is the absorbance of the sample solution before incubation. ANTIOXIDANT ACTIVITY ASSAYS Reducing power. Reducing power was determined according to the method of Oyaizu (17). Three kinds of pearl powders were mixed with 10 ml of DMSO to prepare samples with weight-to-volume ratios of 1, 5, 10, and 20 mg/ml. Two millilitiers of the above samples were then mixed with 2.5 ml of phosphate buffer (0.2 M, pH = 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated in a 50°C water bath for 20 minutes, then rapidly cooled, mixed with 2.5 ml of 10% trichloroacetic acid for fi ve minutes, and centrifuged at 3000 rpm for ten minutes. After centrifugation, 5 ml of the supernatant was mixed with 5 ml of DI water and 1 ml of 0.1% ferric chloride, and this was left to stand for ten minutes. Absorbance at 700 nm was used as the indicator of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)