ALTERNATIVES TO COSMETICS PRESERVATION 115 Anticellulite cream Cleansing milk Peeling cream Experiment Experiment Experiment TAPC* TAPC* TAPC* (log cfu/g) (log cfu/g) (log cfu/g) No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# 5.6 5.67 5.68 5.65 5.7 5.69 5.77 5.72 5.55 5.78 5.68 5.7 0.85 0.92 0.93 0.9 1.8 1.95 1.95 1.9 1.65 1.72 1.43 1.6 0.82 0.91 0.82 0.85 0.94 0.94 0.97 0.95 0.68 0.67 0.75 0.7 0.71 0.7 0.69 0.7 0.85 0.93 0.92 0.9 0.85 0.93 0.92 0.9 0.71 0.7 0.69 0.7 0.71 0.7 0.69 0.7 0.85 0.93 0.92 0.9 Anticellulite cream Cleansing milk Peeling cream Experiment Experiment Experiment TAPC* TAPC* TAPC* (log cfu/g) (log cfu/g) (log cfu/g) No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# 5.53 5.69 5.73 5.65 5.69 5.72 5.69 5.7 5.71 5.69 5.61 5.7 0.85 0.93 0.92 0.9 1.8 1.95 1.95 1.9 1.65 1.72 1.43 1.6 0.82 0.91 0.82 0.85 0.94 0.94 0.97 0.95 0.71 0.68 0.71 0.7 0.71 0.68 0.71 0.7 0.85 0.93 0.92 0.9 0.85 0.93 0.92 0.9 0.48 0.55 0.47 0.5 0.71 0.7 0.69 0.7 0.9 0.83 0.67 0.8 Anticellulite cream Cleansing milk Peeling cream Experiment Experiment Experiment TAPC* TAPC* TAPC* (log cfu/g) (log cfu/g) (log cfu/g) No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# 5.5 5.8 5.26 5.52 5.55 5.78 5.68 5.67 5.5 5.67 5.39 5.5 1.9 1.82 1.83 1.85 1.6 1.53 1.37 1.5 1.85 1.91 1.94 1.9 0.85 0.93 0.92 0.9 0.85 0.92 0.93 0.9 0.85 0.92 0.93 0.9 0.82 0.91 0.82 0.85 0.84 0.9 0.96 0.9 0.68 0.72 0.7 0.7 0.8 0.82 0.72 0.78 0.82 0.88 0.85 0.85 0.77 0.69 0.64 0.7 Table VIa (cont’d) Table VIb (cont’d) Table VIc (cont’d)
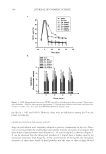
JOURNAL OF COSMETIC SCIENCE 116 emulsifi ed formulations (Figure 1c, Tables IV and VIc). Preservative systems VI and VII revealed excellent activity against this microorganism in the O/W formulations tested, i.e., the anticellulite cream, cleansing milk, and peeling cream. System VIII, with the reduced percentage of levulinic acid, was suffi cient as well. The strain was not detected in the intact product (Tables V and VII). Furthermore, the strain was not recovered during the in-use consumer use test (Tables V and VIII). Aspergillus niger. System I preserved marginally the tonic lotion against this mold in the challenge test, since only criterion B of E. Ph. was achieved (Figure 2 and Table IV). Although no contamination of the mold was detected in the intact product (Table VII), recovery was observed during the in-use study (Table VIII). Addition of (0.1% w/w) p-anisic acid (system II) or (0.3% w/w) levulinic acid (system III) or (5% w/w) ethanol (system IV) to Lonicera extracts (system I) enhanced the effi cacy, and the resulting prod- ucts were microbiologically safe either in the challenge test (Figure 2 and Table IV) or Table VIe Challenge Test of Cosmetic Products Performed in Triplicate Regarding Candida albicans (system V) Shampoo Shower gel Conditioning cream C. albicans Experiment Experiment Experiment TAPC* TAPC* TAPC* (log cfu/g) (log cfu/g) (log cfu/g) No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# Days 0 5.28 5.31 5.31 5.3 5.71 5.76 5.78 5.8 5.29 5.31 5.42 5.3 2 1.75 1.85 1.8 1.8 1.94 1.95 2.05 2 1.87 1.97 2.1 2 7 0.49 0.49 0.52 0.5 0.84 0.9 0.96 0.9 0.93 0.94 0.98 1 14 0.49 0.49 0.52 0.5 0.8 0.9 0.7 0.8 0.9 0.83 0.67 0.8 28 0.31 0.3 0.29 0.3 0.37 0.41 0.42 0.4 0.9 0.83 0.67 0.8 * TAPC = total aerobic plate count. # MV = mean value. Table VId Challenge Test of Cosmetic Products Performed in Triplicate Regarding Aspergillus niger (system V) Shampoo Shower gel Conditioning cream A. niger Experiment Experiment Experiment TAPC* TAPC* TAPC* (log cfu/g) (log cfu/g) (log cfu/g) No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# No.1 No.2 No.3 MV# Days 0 5.6 5.67 5.68 5.65 5.49 5.58 5.43 5.5 5.25 5.32 5.33 5.3 2 0.85 0.93 0.92 0.9 0.8 0.9 0.7 0.8 0.71 0.7 0.69 0.7 7 0.71 0.7 0.69 0.7 0.7 0.9 0.8 0.8 0.71 0.7 0.69 0.7 14 0.62 0.6 0.58 0.6 0.57 0.59 0.64 0.6 0.59 0.6 0.61 0.6 28 0.51 0.51 0.48 0.5 0.38 0.41 0.41 0.4 0.49 0.49 0.52 0.5 * TAPC = total aerobic plate count. # MV = mean value.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)