J. Cosmet. Sci., 61, 125–132 (March/April 2010) 125 Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fi brillar collagens, elastin, and fi brillins by xanthohumol NEENA PHILIPS, MATHEW SAMUEL, ROSEMARIE ARENA, YU-JUN CHEN, JENNIFER CONTE, PRASHANTI NATRAJAN, GERHARD HAAS, and SALVADOR GONZALEZ, School of Natural Sciences, Fairleigh Dickinson University, Teaneck, NJ (N.P., M.S., R.A., Y.-J.C., J.C., P.N., G.H.), Industrial Cantabria Farmaceutica, S.A, Madrid, Spain (S.G.), and Dermatology Service, Memorial Sloan- Kettering Cancer Center, New York (S.G.). Accepted for publication August 24, 2009. Synopsis In skin aging there is deterioration of the extracellular matrix’s collagen and elastin fi bers, from its reduced biosynthesis and increased degradation by elastase and matrixmetalloproteinases (MMPs). Xanthohumol is a fl avonoid isolated from the hop plant Humulus lupulus L., with anti-microbial, antioxidant, anti-infl ammatory, and anti-carcinogenic properties. The goal of this research was to investigate xanthohumol as an anti-skin- aging agent via its benefi cial regulation of the extracellular matrix. To this purpose, we examined the direct effect of xanthohumol on the activities of elastase and MMPs (MMPs 1, 2, and 9) and its effect on the expres- sion (protein and/or transcription levels) of collagens (types I, III, and V), elastin, and fi brillins (1 and 2) in dermal fi broblasts. Xanthohumol signifi cantly inhibited elastase and MMP-9 activities from its lowest con- centration, and MMP-1 and MMP-2 at its higher concentrations, which implies a greater protective effect on elastin. It dramatically increased the expression of types I, III, and V collagens, and elastin, fi brillin-1, and fi brillin-2 in dermal fi broblasts. The effects were similar to those of ascorbic acid. This is the fi rst report identifying xanthohumol’s potential to improve skin structure and fi rmness: it simultaneously inhibits the activities of elastase/MMPs and stimulates the biosynthesis of fi brillar collagens, elastin, and fi brillins. INTRODUCTION Alterations in collagen and elastin, which form the extracellular matrix (ECM), are re- sponsible for the clinical manifestations of skin aging, which are wrinkles, sagging, and laxity (1–6). The fi brillar collagens, types I, III, and V, in the order of predominance, provide structure whereas elastin forms elastic fi bers with fi brillin to gives skin fi rmness and elasticity. The atrophy of collagen and elastin fi bers in skin aging is from reduced Address all correspondence to Neena Philips.
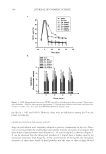
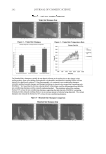
JOURNAL OF COSMETIC SCIENCE 126 synthesis, and the increased expression of their degradative enzymes, especially collage- nases (MMP-1), gelatinases (MMP-2, MMP-9), and elastases (1–14). The cosmetic industry is active in identifying natural products to counteract skin aging. Many of these products or actives have antioxidant and/or anti-infl ammatory properties, and are rich in polyphenols or fl avonoids (15–26). A fl avonoid that has been extensively investigated for its antimicrobial and anticarcinogenic properties, but not for its anti- skin-aging potential, is xanthohumol from the hop plant Humulus lupulus L. (Cannabi- naceae) (hops) (27–31). It has potent anti-infl ammatory and antioxidant properties, including inhibition of the NF-kB transcription factor and nitric oxide production by infl ammatory cytokines (27–31). Xanthohumol’s potential for anti-skin-aging has not been reported. We evaluated the effi cacy of xanthohumol in (a) inhibiting elasatase, MMP-1, MMP-2, and MMP-9 activ- ities (b) stimulating expression of types I, III, and V collagens, elastin, fi brillin-1, and fi brillin-2. MATERIALS AND METHODS MATERIALS The materials used were the following: enzymes [elastase (Elastin Products Co. SE563), MMP-1 (Biomol Se-180), MMP-9 (Biomol Se-244), MMP-2 (Biomol Se-109)] substrates [elastase (Bachem I-1270), MMP-1 (Bachem, M-2055), MMP-2,9 (Bachem M-1855)] dermal fi broblasts (Cascade Biologics) escort (Sigma) protein detector kit (KPL Co) antibodies/standards [collagen (Chemicon), elastin (Elastin Products Co)] plasmids (gifts from Dr. Joel Rosenbloom, School of Dental Medicine, University of Pennsylvania, PA) dual luciferase reporter assay (Promega) xanthohumol (gift from Dr. Gearhaard Haas, Fairleigh Dickinson University, NJ) and positive controls [ascorbic acid (AA, Sigma), EDTA (Sigma), PMSF (Sigma), protease inhibitor (Roche)] ELASTASE OR MMP ACTIVITY CALIBRATION AND INHIBITION The enzymes (elastase, MMP-1, MMP-9, and MMP-2) were calibrated by reacting two- fold serial dilutions of each of the enzymes (starting concentration of 1 μg/μl) with respec- tive substrates (0.5 mM) in incubation buffer (elastase: 0.09 M Tris-0.5 M NaCl buffer MMPs: 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM CaCl2). The reaction kinetics was measured fl uorometrically (355 excitation/450 emission) every fi ve minutes for a total length of 1.5 hours. The optimal enzyme concentrations (linear dose response) were determined to be 0.1 μg/ml for elastase, 0.77 μg/μl for MMP-1, 0.5 μg/μl for MMP-2, and 0.1 μg/μl for MMP-9. Xanthohumol (0.001%, 0.01%, 0.1%, and 1% of a stock of 50 mg/ml) (Xan) or positive controls (10 mM PMSF, 5m MEDTA, 0.5 mM ascorbic acid or 1XProtease inhibitor) were incubated with the optimal concentration of each of the enzymes in incubation buffer for ten minutes followed by the addition of the respective substrate. The activities of elastase and each of the MMPs were measured fl uorometrically at 355 excitation/450 emission.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)