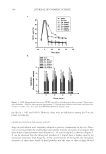
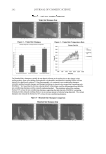
SUNLESS TANNING FORMULATIONS 75 IN VITRO/IN VIVO EFFICACY EVALUATIONS—GENERAL APPROACH An in vitro effi cacy testing methodology for evaluation of sunless tanners described by Jermann et al. (6) with our modifi cations (7) was utilized pre-hydrated Vitro Skin® (N-19) (8) was used as a substrate. This in vitro methodology is a reliable tool to predict the effi cacy and differences of self-tanning formulation performance on human skin (5–7). For the analysis of skin color in vivo after the application of test articles, the “natural uni- verse of suntan” and “natural universe of suntan tonality” realms were from Muizzuddin et al. (9), describing “…a cluster plane encompassing the distribution of normal skin tan- ning color representing the ‘natural universe’ of skin tanning or a response region within which natural skin tan color was observed.” PROCEDURE—IN VITRO The substrate was pre-cut into 4-cm by 4-cm pieces and pre-hydrated according to IMS-USA protocol (8). The application dose was 2 mg/cm2 temperature: 76°–78°F. Each piece of substrate was prepared for the experiment by uniformly applying 0.032 grams (2 mg/cm2) of test article on the surface with a pre-saturated fi nger cot. The substrate was then placed in a slide frame and put in the color development chamber. Three slides for each test article were used. The test articles were coded and their compositions were revealed after the in vitro study was completed. The colors of the samples were evaluated every 24 hours (three measurements per slide) for four days with a ColorTec-PSMTM Colorimeter: Observer 10° primary illuminant D65 CIE (1976) L*a*b* color space with tri-stimulus color values: L* (lightness), a* (red-green axis), and b* (yellow-blue axis). The difference in C* (chroma/saturation) is calculated according to the following equation: dC da db * ( * * ) = + 2 2 Table I Description of Test Formulations: X(with cationic polymer-based rheology modifi er) and K (with polyacrylamide-based rheology modifi er) %w/w Ingredient: INCI name X K Water 89.65 89.65 Pentylene glycol 4.00 4.00 Dihydroxyacetone 3.00 3.00 Phenoxyethanol (and) methylparaben (and) ethylparaben (and) butylparaben (and) propylparaben (and) isobutylparaben 0.35 0.35 Dimethylacrylamide/ethyltrimonium chloride methacrylate copolymer (and) propylene glycol dicaprylate/dicaprate (and) PPG-1 trideceth-6 (and) C10-11 isoparaffi n 3.00 — Polyacrylamide (and) C13-14 isoparaffi n (and) laureth-7 — 3.00 Sodium hydroxide, 10% aqueous solution q.s. q.s. pH 3.90 3.76
JOURNAL OF COSMETIC SCIENCE 76 The total difference between two colors in CIE space is described as ΔE* ab and provides an integrated measurement of both chroma and lightness/darkness changes: dE ab dL da db * [( * ) * ) ( * )] = 2 2 2 PROCEDURE—IN VIVO In vivo studies were conducted on fi ve panelists using the volar aspects of their forearms as application sites. Application areas were 50 cm2. Before application of the test formula- tions, all panelists washed their forearms with Dove bar soap, rinsed with water, and pat- ted their forearms dry with paper towels. A period of 15 minutes was allowed before conducting the initial measurement of the skin color on marked application areas (fi ve measurements per area) using the ColorTec-PSM Colorimeter. The test articles were coded to hide their identity from those used in the in vitro studies, and their compositions were disclosed after the in vivo study was completed. Each test article was applied once on each panelist. The application dose was 2 mg/cm2, with an exact application amount of 0.1 g per application area. Left and right forearms were alternated to randomize the ap- plication sites for the test articles. The products were applied using fi nger cots pre-satu- rated with test article. Color measurements were taken at 24, 48, and 120 hours after a single application. Between measurements panelists were asked to follow their regular hygiene routine, taking showers, washing hands, etc. MATERIALS AND EQUIPMENT USED IN THE IN VITRO/IN VIVO STUDIES • Vitro-Skin® (N-19) (Lot# 8302), foam block and glassless slide mounts from IMS, Inc. • Powder-free class 100 fi nger cots (Lot# FOY8) from Fisher Scientifi c • ColorTec-PSM Colorimeter from ColorTec. HPLC METHOD An analytical method for the determination of the concentrations of DHA in sunless tanning formulations was developed based on the method described by Biondi et al., HPLC analysis of a pentafl uorobenzyloxime derivative (10) with our modifi cations. Sample preparation for HPLC. The samples were prepared for analytical testing by combin- ing 0.25 grams of each sample with 5 ml of sodium chloride (NaCl)-saturated aqueous solution, followed by vortex mixing for one minute. Then 5 ml of water was added to each sample and stirred for six hours. After that 25 μl of the sample and 50 μl of deriva- tizing agent O-(2, 3, 4, 5, 6-pentafl uorobenzyl) hydroxylamine hydrochloride 98% (Aldrich, 194484) were pipetted into a 10-ml volumetric fl ask and diluted using 50:50 acetonitrile and water. The standard used was DHA, cosmetic grade, from Napp Technologies LLC (Lot LL07- 2046). The preparation consisted in dissolving 0.0330 grams of the reagent in 10 ml of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)