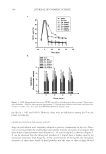
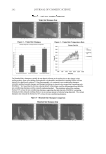
COMPARISON OF DIFFERENT PEARL POWDERS 143 can be introduced to minimize the effects of ROS, and natural antioxidants are of high interest. Reducing power can be attributed mainly to the bioactive compounds associated with antioxidant activity. These bioactive compounds include the vitamin E derivative Trolox, fl avonoids from the bark of Pinus Pinaster Pycnogenol, the leaves of Ginkgo biloba (EGb 761) (30), and pyridine derivatives that were originally isolated from the fresh roots of Taraxacum (31). This study show that P-w, P-μ, P-n, ginkgo, and Tara have been shown to be good electron donors and could terminate radical chain reactions by converting free radicals to more stable products. DPPH has been widely used to evaluate the free-radical scavenging effects of various an- tioxidant substances. Unsaturated lipids in cell membranes are susceptible to peroxida- tion. This chain reaction is initiated by hydroxyl radicals attacking lipids, and it is extended by the generated lipid hydroperoxide free radicals. It has been reported that extract of ginseng exhibits scavenging activity against DPPH radicals in vitro, and that this extract shows scavenging activity for hydroxyl radicals to prevent lipid peroxidation (32). In this study, the three kinds of pearl powders and ginkgo showed similar scaveng- ing activity against DPPH radicals, and the free-radical scavenging effects of P-μ and P-n were greater than those of P-w. Iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The ferrous state of iron accelerates lipid oxidation by breaking down hydrogen and lipid peroxides into reactive free radicals. In the presence of chelating agents, the complex forma- tion is disrupted, resulting in a decrease in oxidation reactions. It is reported that the metha- nolic extracts (MEs) of various processed tomatoes at a concentration of 2 mg/ml could reach more than 65% ferrous ion chelating ability (FICA). The use of soybean sprout extract at a concentration of 3 mg/ml was required to obtain the same level of FICA. MEs from me- dicinal mushroom of Chang-chih could reach a FICA of 64.4–74.5% at 5 mg/ml (33). In comparison, three kinds of pearl powders at a concentration of 1 mg/ml reach a FICA of 80%. CONCLUSIONS From the above, it can be understood that the pearl powder has fi ne moisturizing effi ciency for human skin. It can improve not only the barrier action for the moisturization of the skin but also the hydration of the skin. P-μ, in particular, has better hydration than P-w. The three kinds of pearl powders can also signifi cantly inhibit the activation of tyrosinase. Moreover, the three kinds of pearl powders have strong capacities for restraining and re- moving free radicals. More remarkably, in tests of reducing power and DPPH for scaveng- ing free radicals, P-μ and P-n showed better performance than P-w, whereas P-n showed better performance than P-μ. These results provide suffi cient scientifi c proof for the effi - cacy of pearl powders for beauty treatments, resistance to aging, and clinical treatments, among other applications, and help us to use natural pearl powders to benefi t the people. REFERENCES (1) D. Tsukamoto, I. Sarashina, and K. Endo, Structure and expression of an unusually acidic matrix protein of pearl oyster shells, Biochem. Biophys. Res. Commun., 320, 1175–1180 (2004).
JOURNAL OF COSMETIC SCIENCE 144 (2) C. Zhang, L. Xie, J. Huang, X. Liu, and R. Zhang, A novel matrix protein family participating in the prismatic layer framework formation of pearl oyster, Pinctada fucata, Biochem. Biophys. Res. Commun., 344, 735–740 (2006). (3) L. H. Zhang, J. L. Wang, H. P. Mei, and B. Luo, Beauty effect and health care effi cacy of pearl, Deterg. Cosmet., 25, 177–180 (2002). (4) C. P. Suja and S. Dharmaraj, In vitro culture of mantle tissue of the abalone Haliotis varia Linnaeus, Tissue Cell., 37, 1–10 (2005). (5) Y. Tokiwa, M. Kitagawa, and T. Raku, Enzymatic synthesis of arbutin undecylenic acid ester and its inhibitory effect on mushroom tyrosinase, Biotechnol. Lett., 29, 481–486 (2007). (6) J. Busciglio and B. A. Yankner, Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro, Nature, 378, 776–779 (1995). (7) G. R. Zhao, Z. J. Xiang, T. X. Ye, Y. J. Yuan, and Z. X. Guo, Antioxidant activities of Salviamiltior- rhiza and Panax notoginseng, Food Chem., 99, 767–774 (2006). (8 B. H. Havsteen, The biochemistry and medical signifi cance of the fl avonoids, Pharmacol. Ther., 96, 67–202 (2002). (9) T. S. Wu, C. C. Hwang, P. C. Kuo, T. H. Kuo, A. G. Damu, and C. R. Su, New neolignans from Spiraea formosana, Chem. Pharm. Bull., 52, 1227–1230 (2004). (10) L. J. Meyer and R. Stern, Age-dependent changes of hyaluronan in human skin, J. Invest. Dermatol., 102, 385–389 (1994). (11) H. Scholtyssek, W. Damerau, R. Wessel, and I. Schimkek, Antioxidative activity of ginkgolides against superoxide in an aprotic environment, Chem. Biol. Interact., 106, 183–190 (1997). (12) H. J. Jeon, H. J. Kang, H. J. Jung, Y. S. Kang, C. J. Lim, Y. M. Kim, and E. H. Park, Anti-infl ammatory activity of Taraxacum offi cinale, J. Ethnopharmacol., 115, 82–88 (2008). (13) K. K. Song, L. Qiu, H. Huang, and Q. X. Chen, The inhibitory effect of tyrosinase by arbutin as cos- metic additive, J. Xiamen. Univ., 42, 791–794 (2003). (14) X. L. Piao, S. H. Baek, M. K. Park, and J. H. Park, Tyrosinase-inhibitory furanocoumarin from An- gelica dahurica, Biol. Pharm. Bull., 27, 1144–1146 (2004). (15) Y. V. Yuan and N. A. Walsh, Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds, Food Chem. Toxicol., 44, 1144–1150 (2006). (16) S. Rout and R. Banerjee, Free radical scavenging, anti-glycation and tyrosinase inhibition properties of a polysaccharide fraction isolated from the rind from Punica granatum, Bioresour. Technol., 98, 3159– 3163 (2007). (17) Y. L. Lee, M. T. Yen, and J. L. Mau, Antioxidant properties of various extracts from Hypsizigus mar- moreus, Food Chem., 104, 1–9 (2007). (18) K. Shimada, K. Fujikawa, K. Yahara, and T. Nakamura, Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion, J. Agric. Food Chem., 40, 945–948 (1992). (19) T. C. Dinis, V. M. Maderia, and L. M. Almeida, Action of phenolic derivatives (acetaminophen, salicy- late, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scaven- gers, Arch. Biochem. Biophys., 315, 161–169 (1994). (20) P. G. Sator, J. B. Schmidt, and H. Honigsmann, Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis, J. Am. Acad. Dermatol., 48, 352– 358 (2003). (21) S. A. Wissing and R. H. Muller, The infl uence of solid lipid nanoparticles on skin hydration and vis- coelasticity—in vivo study, Eur. J. Pharm. Biopharm., 56, 67–72 (2003). (22) Y. Sunwoo, C. Chou, J. Takeshita, M. Murakami, and Y. Tochihara, Physiological and subjective re- sponses to low relative humidity, J. Physiol. Anthropol., 25, 7–14 (2006). (23) H. Tagami, K. Yoshikuni, K. Inoue, M. Yamada, and Y. Iwase, Functional analysis of factors infl uencing the water content of the stratum corneum in vivo, Nippon. Hifuka. Gakkai. Zasshi., 92, 1363–1367 (1982). (24) A. Sanchez-Ferrer, J. N. Rodriguez-Lopez, J. N. Garcia-Canovas, and F. Garcia-Carmona, Tyrosinase: A comprehensive review of its mechanism, Biochim. Biophys. Acta, 1247, 1–11 (1995). (25) I. Kubo, Y. Yokokawa, and I. Kinst-Hori, Tyrosinase inhibitors from Bolivian medicinal plants, J. Nat. Prod., 58, 739–743 (1995). (26) Y. Shi, Q. X. Chen, Q. Wang, K. K. Song, and L. Qiu, Inhibitory effects of cinnamic acid and its deriv- atives on the diphenolase activity of mushroom (Agaricus bisporus) tyrosinase, Food Chem., 92, 707–712 (2005). (27) K. Maeda and M. Fukuda, In vitro effectiveness of several whitening cosmetic components in human melanocytes, J. Soc. Cosmet. Chem., 42, 361–368 (1991).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)