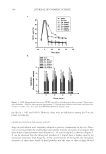
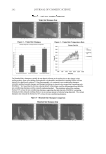
JOURNAL OF COSMETIC SCIENCE 130 associated with drastic deterioration of the structural ECM (1,2,12,13,15,18). The der- mal fi broblasts are the predominant cells responsible for the alterations in the ECM. Hence the effects of xanthohumol on the expression of ECM proteins were examined in these cells. Xanthohumol drastically increased expression of fi brillar collagen and elastin fi ber com- ponents, especially at 0.1%, where the effect was similar to that of ascorbic acid for col- lagens and about fourfold greater than the effect of ascorbic acid on elastin and fi brillin expression (Figure 2). Xanthohumol signifi cantly increased type I collagen expression transcriptionally in fi bro- blasts at 1% (protein: 224% of control promoter: 449% of control) and 0.1% (protein: 578% of control promoter: 1347% of control) (p0.05) (Figure 2a). Relative to the con- trol (0 at 100%), xanthohumol at 1% and 0.1%, respectively, signifi cantly stimulated the protein levels of type III collagen to 745% and 1390% of the control and type V collagen to 304% and 893% of the control (p0.05) (Figure 2a). Elastin expression was signifi cantly stimulated at the transcriptional level by xantho- humol at 1% (protein to 218% and promoter to 367%) and 0.1% (protein to 692% and promoter to 1288% relative to control) (Figure 2b). Xanthohumol at 0.1% and 1% sig- nifi cantly increased the expression of fi brillin-1 to 1823% and 603%, respectively, relative to the control, and fi brillin-2 expression to 1713% and 373% of the control, respectively (p0.05) (Figure 2b). CONCLUSION Xanthohumol has antioxidant and anti-infl ammatory properties, and thereby its po- tential for anti-skin-aging was investigated in this research. The targets examined were the extracellular matrix remodeling and structural proteins: elastase, MMP-1, MMP-2, MMP-9, types I, III, and V collagens, elastin, fi brillin-1, and fi brillin-2. Xanthohumol demonstrated dual protective effects on the extracellular matrix via the inhibition of the ECM proteolytic enzymes and the stimulation of the structural ECM components, collagens, elastin, and fi brillins. We identify xanthohumol as an anti-skin-aging agent via its benefi cial regulation of the ECM. The intake or topical application of xanthohumol may be benefi cial to skin health and preferred over hor- mones with similar effects (19). ACKNOWLEDGMENTS The authors thank Tiffany Chang, Joshua Givant, Sujini Yarlagadda, Marvin Tuason, and May Taw for their assistance. REFERENCES (1) M. H. Shin, G. E. Rhie, C. H. Park, K. H. Kim, K. H. Cho, H. C. Eun, and J. H. Chung, Modulation of collagen metabolism by the topical application of dehydroepiandrosterone to human skin, J. Invest. Dermatol., 124, 315–323 (2005). (2) J. Uitto, M. J. Fazio, and D. Olsen, Molecular mechanisms of cutaneous aging, J. Am. Acad. Dermatol., 21, 614–622 (1989).
REGULATION OF ECM REMODELING BY XANTHOHUMOL 131 (3) J. Varani, R. L. Warner, and M. Gharaee-Kermani, Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in natu- rally aged human skin, J. Invest. Dermatol., 114, 480–486 (2000). (4) C. Lee, H. Choi, H. Kim, D. Kim, I. Chang, H. T. Moon, S. Lee, W. K. Oh, and E. Woo, Bifl avonoids isolated from Selaginella tamariscina regulate the expression of matrix metalloproteinase in human skin fi broblasts, Bioorg. Med. Chem., 16, 732–738 (2008). (5) M. R. Khorramizadeh, E. E. Tredget, C. Telasky, Q. Shen, and A. Ghahary, Aging differentially modu- lates the expression of collagen and collagenase in dermal fi broblasts, Mol. Cell. Biochem., 194, 99–108 (1999). (6) A. J. Millis, M. Hoyle, H. M. McCue, and H. Martini, Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fi broblasts, Exp. Cell. Res., 201, 373–379 (1992). (7) A. Berton, G. Godeau, H. Emonard, K. Baba, P. Bellon, W. Hornebeck, and G. Bellon, Analysis of the ex vivo specifi city of human gelatinases A and B towards skin collagen and elastic fi bers by computer- ized morphometry, Matrix Biology, 19, 139–148 (2000). (8) M. Brennan, H. Bhatti, K. C. Nerusu, N. Bhagavathula, S. Kang, G. J. Fisher, J. Varani, and J. J. Voor- hees, Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin, Photochem. Photobiol., 78, 43–48 (2003). (9) N. Tsuji, S. Moriwaki, Y. Suzuki, Y. Takema, and G. Imokawa, The role of elastases secreted by fi bro- blasts in wrinkle formation: Implication through selective inhibition of elastase activity, Photochem Pho- tobiol., 71, 283–290 (2001). (10) F. Rijken, R. C. Kiekens, E. van den Worm, P. L. Lee, H. van Weelden, and P. L. Bruijnzeek, Pathophys- iology of photoaging of human skin: Focus on neutrophils, Photochem. Photobiol. Sci., 5, 184–189 (2006). (11) F. Rijken, R. C. Kiekens, and P. L. Bruijnzeek, Skin-infi ltrating neutrophils following exposure to solar- simulated radiation could play an important role in photoageing of human skin, Br. J. Dermatol., 152, 321–328 (2005). (12) E. Jung, J. Lee, J. Baek, K. Jung, J. Lee, S. Huh, S. Kim, J. Koh, and D. Park, Effect of Camellia japon- ica oil on human type I procollagen production and skin barrier function, J. Ethnopharmacol., 112, 127–131 (2007). (13) H. S. Cho, M. H. Lee, J. W. Lee, K. O. No, S. K. Park, H. S. Lee, S. Kang, W. G. Cho, H. J. Park, K. W. Oh, and J. T. Hong, Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation, Photodermatol. Photoimmunol. Photomed., 23, 155–162 (2007). (14) J. Labat-Robert, A. Fourtanier, B. Boyer-Lafargue, and L. Robert, Age dependent increase of elastase type protease activity in mouse skin. Effect of UV-irradiation, J. Photochem. Photobiol B, 57, 113–118 (2000). (15) K. K. Lee and J. D. Choi, The effects of Areca catechu L extract on anti-aging, Int. J. Cosmet. Sci., 21, 285–295 (1999). (16) Y. H. Kim, K. S. Kim, C. S. Han, H. C. Yang, S. H. Park, K. I. Ko, S. H. Lee, K. H. Kim, N. H. Lee, J. M. Kim, and K. H. Son, Inhibitory effects of natural plants of Jeju Island on elastase and MMP-1 expression, J. Cosmet. Sci., 58, 19–33 (2007). (17) K. Tsukahara, H. Nakagawa, S. Moriwaki, Y. Takema, T. Fujimura, and G. Imokawa, Inhibition of ul- traviolet-B-induced wrinkle formation by an elastase-inhibiting herbal extract: Implication for the mechanism underlying elastase-associated wrinkles, Int. J. Dermatol., 45, 460–468 (2006). (18) F. Jimenez, T. F. Mitts, K. Liu, Y. Wang, and A. Hinek, Ellagic and tannic acids protect newly synthe- sized elastic fi bers from premature enzymatic degradation in dermal fi broblast cultures, J. Invest. Derma- tol., 126, 1272–1280 (2006). (19) N. Philips and J. Devaney, Benefi cial regulation of type I collagen and matrixmetalloproteinase-1 ex- pression by estrogen, progesterone and its combination in skin fi broblasts, J. Am. Aging Assoc., 26, 59– 62 (2004). (20) N. Philips, J. Smith, T. Keller, and S. Gonzalez, Predominant effects of Polypodium leucotomos on mem- brane integrity, lipid peroxidation, and expression of elastin and matrixmetalloproteinase-1 in ultravio- let radiation exposed fi broblasts, and keratinocytes, J. Dermatol. Sci., 32, 1–9 (2003). (21) N. Philips, T. Keller, C. Hendrix, S. Hamilton, R. Arena, M. Tuason, and S. Gonzalez, Regulation of the extracellular matrix remodeling by lutein in dermal fi broblasts, melanoma cells, and ultraviolet ra- diation exposed fi broblasts, Arch. Dermatol. Res., 299, 373–379 (2007).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)