J. Cosmet. Sci., 64, 235–241 ( July/August 2013) 235 Kinetics of inhibitory effect of isoferulic acid on mushroom tyrosinase SHENGZHAO GONG, MEIJUAN YIN, and ZHIMIAN YUN, Department of Chemical Engineering, Guangdong Industry Technical College, Guangzhou, China 510300. Accepted for publication January 31, 2013. Synopsis A study on the kinetics of inhibitory effect of isoferulic acid on the monophenolase and diphenolase activity of mushroom tyrosinase was carried out using enzymological kinetic analysis method in a Na2HPO4– NaH2PO4 buffer solution (pH = 6.8) at 30°C. It was found that isoferulic acid effi ciently inhibits both monophenolase and diphenolase activities of mushroom tyrosinase under experimental conditions. Concen- trations of isoferulic acid leading to 50% rate inhibition (IC50) on monophenolase and diphenolase activity were calculated to be 0.13 mmol/L and 0.39 mmol/L, respectively, which are much lower than that of arbutin (IC50 = 5.3 mmol/L for diphenolase activity). The presence of isoferulic acid also prolongs the lag period in the oxidation process of L -tyrosine via tyrosinase—a 4.3-min lagging was observed with the presence of 0.20 mmol/L isoferulic acid—compared to a 1.1-min lagging in the absence of isoferulic acid. The Lineweaver– Burk plot demonstrates a competitive behavior of isoferulic acid in the tyrosinase oxidation of L -3,4- dihydroxyphenylalanine, with maximum reaction rate (vm) and inhibition constant (KI) at 64.5 μM/min and 0.11 mmol/L, respectively. INTRODUCTION Tyrosinase, also known as polyphenol oxidase, is a copper-containing mixed-function oxidase widely distributed in microorganisms, animals, and plants (1,2). It is recognized as a pivotal enzyme in the process of melanin biosynthesis. Tyrosinase catalyzes two criti- cal reactions in melanin synthesis: (i) hydroxylation of monophenol to o-diphenol (mono- phenolase activity) and (ii) conversion of o-diphenol to the corresponding o-quinone (diphenolase activity). The resulting quinone is subsequently subjected to a series of oxi- dation/polymerization processes to form dark pigments, also known as “melanine.” It is evident that tyrosinase is a key enzyme controlling the melanization process of skin, eye, inner ear, and hair, as well as the enzymatic browning process in fruits and vegetables (3–5). Meanwhile, the application of tyrosinase inhibitors has attracted more and more attention in the fi elds of cosmetic, food, and pharmaceutical industry, primarily because Address all correspondence to Shengzhao Gong at 1996103022@gditc.edu.cn.
JOURNAL OF COSMETIC SCIENCE 236 of their high effi cacy in mitigating hyperpigmentation (6). Much effort has been made in searching for feasible and effective tyrosinase inhibitors. For instance, based on systematic studies on the inhibitory effect of quercetin, dodecyl gallate, and thymol on mushroom tyrosinase, Kubo et al. proposed a kinetic model of the inhibition process and pointed out some favorable features in molecular structure for a potential effective inhibitor (4,6,7). Nerya et al. analyzed a series of inhibitors extracted from the root of Licorice (8). Gong et al. has previously reported some potent tyrosinase inhibitors, such as ferulic acid and cinnamic acid (9,10). Isoferulic acid is an active component found in Rhizoma Cimicifugae. Reported in this paper is a kinetic study on the inhibitory effect of isoferulic acid on mushroom tyrosinase. By investigating the in vitro inhibitory effect of isoferulic acid on both monophenolase and diphenolase activities of mushroom tyrosinase, a competitive inhibition model was established and the kinetic parameters were calculated. The current results provide ex- perimental support for the potential application of isoferulic acid as a high-effi cacy anti- pigment ingredient in industry. EXPERIMENTAL MATERIALS Mushroom tyrosinase, L -tyrosine, and L -3,4-dihydroxyphenylalanine (L-DOPA) were pur- chased from Sigma (Shanghai, China). Isoferulic acid was obtained from the National Institute for the Control of Pharmaceutical and Biological Product (Guangdong, China). Dimethyl sulfoxide (DMSO) and other reagents were of analytical grade and obtained from com- mercial suppliers. Double distillated and de-ionized water was used unless stated otherwise. METHODS The diphenolase activity assay was performed as previously reported (9). The monophe- nolase activity assay was performed with L -tyrosine as substrate. Using a microsyringe, a tyrosinase solution (0.20 ml, 0.38 mmol/L) was added to a thermostatic solution (5.0 ml, 30°C) containing 50 mmol/L Na2HPO4–NaH2PO4 (pH = 6.8), 1.5 mmol/L L -tyrosine and different concentrations of isoferulic acid (predissolved in DMSO). The resulting mixture was mixed thoroughly using the syringe and immediately monitored by spectro- photometry at 475 nm for 10 min. The molar absorption coeffi cient of the oxidation product (o-quinone) from the substrate (L-tyrosine or L -DOPA) is calculated to be 3700 (mmol/L·cm)-1. Absorption data were recorded on an HP 6010 UV spectrophotometer by Agilent Technologies (Shanghai), China. RESULTS AND DISCUSSION EFFECT OF ISOFERULIC ACID CONCENTRATION ON MONOPHENOLASE ACTIVITY OF TYROSINASE The inhibitory effects of different concentrations of isoferulic acid on the oxidation of L -tyrosine via tyrosinase were studied. The kinetic course of the oxidation of L -tyrosine in
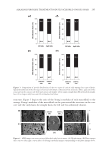
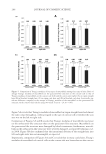
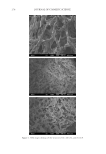
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)