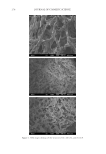
GCL/GCP GELS ENHANCE SKIN DELIVERY OF MAP 277 RESULTS AND DISCUSSION CHARACTERIZATION OF SYNTHESIZED GCP/GCL Both the freeze-dried GCL/GCP appeared to be a spongy, porous white solid. SEM images revealed the gels to have an extremely porous structure (Figure 1). GCL and GCP analyzed using FTIR showed absorption bands characteristic of amide I and II bands around 1650 and 1550 cm-1. The 2860–2930 cm-1 aliphatic C–H stretching bands of GCL/GCP were attenuated in comparison with lauric and palmitic acids, but were stronger than GC. The FTIR spectra confi rmed the attachment of aliphatic group to GC to form GCL (Supplemen- tary Figure 1)/GCP (Supplementary Figure 2). The relative molecular weights of GCL and GCP were 124 and 140 kDa as determined by GPC, respectively. The level of lauroylation in GCL and palmitoylation in GCP estimated by 1 H NMR spectroscopy was found to be 16.3 ± 2.5 (n = 10) and 13.4 ± 1.8 (n = 7) mole %, respectively. The viscosity of 1% GC, GCL, and GCP prepared in either Milli-Q water or 10% ethanol was comparable however, viscosity of GCP was slightly higher than GCL, and Milli-Q water gel was more viscous than 10% ethanol gel (Table I). When GCL/GCP concentration in the gel was increased from 1% to 3%, the viscosity was also increased less than proportionally. The presence of 3% MAP in the gels did not change the viscosity signifi cantly (data not shown). The physicochemical properties of the synthesized GCL/GCP were similar to those previ- ously reported (11,12). It is known that properties of polymers are directly related to the structure of the cross-link network. The higher level of lauroylation in GCL may be ex- plained by the greater reactivity of shorter chain length fatty acid, as inferred from the report by Dey (16), molar cross-link density of polymer network increased with decreas- ing fatty acid chain length for the preparation of vinyl ester resins containing methacry- lated fatty acid comonomer. Lower cross-link density of GCP may result in a more porous network structure, as suggested by Raj Singh et al. (17). The slight difference in gel vis- cosity may be due to higher molecular weight of GCP than GCL, as indicated by Zlatanic ´ et al. (18) that viscosity was signifi cantly infl uenced by molecular weight of the polymer. Viscosity of polymers containing both hydrophobic and hydrophilic structures is dependent on polymer composition and concentration, but solvent also plays an impor- tant role (19). It was shown that incorporation of ethanol in aqueous solutions of polyvi- nyl alcohol and methylcellulose improves their salvation and inhibit gelation, and addition of alcohol to cellulose acetate and acidic chitosan solutions reduces the viscosity and increases the homogeneity (20,21). MAP RELEASE TEST MAP release profi les from GCL/GCP gels as a function of time were shown in Figure 2, and their release rates were summarized in Figure 3 and Table I. Release rate of MAP from 1% GC aqueous gel was similar to aqueous solution, whereas that from 1% GCL and GCP gels was signifi cantly reduced to 65% and 70% of aqueous solution. Release rate was further slightly reduced when the concentration of GCL/GCP was increased from 1% to 3%. When prepared in 10% ethanol, MAP release from 1% GC was 19.5% higher than from 10% ethanol solution, but that from 1% GCL and 1% GCP was comparable to from 10% ethanol solution, and were 13.2% and 24.4% lower than from 1% GC, all
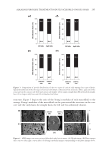
JOURNAL OF COSMETIC SCIENCE 278 Figure 1. SEM images showing (A) the structure of GC, (B) GCL, and (C) GCP.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)