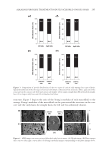
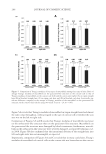
DISPARATE SPF TESTING METHODOLOGIES 305 Figure 3. Correlation analysis of mean SPF values produced by FDA-FM and the Aus/NZ Method. Figure 2. Correlation analysis of mean SPF values produced by international method and the FDA-FM. a strong positive correlation between each pair. The differences in least square mean SPF for each method pair were 0.12, 0.62, and 0.81, respectively these differences also show no statistically signifi cant differences between the mean SPFs obtained using the different testing methods. The use of 25% exposure increments or 15% exposure increments produce similar SPF values when utilizing the same test method (12). Our data illustrate that incremental doses in irradiation sites, ranging from 12% to 25%, in combination with the other methodology variables existing between the FDA-FM method, the Aus/NZ method, and the International method, have no signifi cant impact on the mean SPF value produced. Likewise, statistically equivalent SPF values are produced by 10 subject trials as by 20 subject trials. Furthermore, using different sunscreen standards in a clinical trial induces no signifi cant change in mean SPF value. We conclude that the procedure discrepancies
JOURNAL OF COSMETIC SCIENCE 306 in FDA-FM method, Aus/NZ Method, and the International method are inconsequen- tial either the differences have no impact on mean SPF value, or, less likely, the differ- ences produce equally and opposite changes in mean SPF, thus cancelling any effects. Marketed sunscreens labeled according to the mean effi cacy value as determined by any of these methods would produce a universally defi nable SPF. However formulations evalu- ated with the International method (5,6) or Aus/NZ Method (4) are designated a label with the mean SPF value when it fi ts within statistical criterion testing for precision and accuracy. In contrast, the FDA-FM (1) method and the more recently published FDA Final Rule (3) subtract an “A” value from the mean SPF to calculate the SPF label. The A value is composed of the product of the upper 5% point of the t-distribution and the standard deviation, divided by √(n), where n equals the number of subjects. The authors are not aware of any other drug that has a clinically determined effi cacy value altered to a different label effi cacy value. This calculation decreases the SPF determined by the FDA-FM and FDA Final Rule to a value that could be statistically different from the SPF value determined by either the International method or the Aus/NZ method, likely re- sulting in identical formulations labeled with different SPF values. Recently, there has been a fl urry of newly published sunscreen standards. The FDA pub- lished the Labeling and Effectiveness Testing: Sunscreen Drug Products for OTC Human Use or Final Rule (3) on June 17, 2011 the International Organization for Standardiza- tion produced a new method (7) and Aus/NZ published a revised method (8) that mir- rors the ISO method (7). While we have no dataset to compare these methods to the FDA-FM method, we know of no reason to believe that these three new methods would produce SPF values with statistically signifi cant differences from the three methods com- pared herein. One notable change implemented by the FDA, reduction of a panel size from 20 to 10, is substantiated by the data presented herein. In conclusion, the contemporary method of being able to sell sunscreen products in all markets requires the concurrent utilization of all three methods. The data presented herein illustrates the equivalency of mean SPFs generated using each method. The differ- ences inherent in each method, such as panel size, time frame for erythemal evaluation, geometric progression of UV dose, SPF of reference sunscreen formulations, and statisti- cal criteria, do not have a signifi cant impact on the mean SPF value produced. These compulsory testing standards are similar enough to render simultaneous use of all three as redundant compliance with one should suffi ce for SPF labeling in all markets. This would reduce the number of subjects experiencing the risks of SPF trials unnecessarily (13) while also bringing the static sunscreen testing methodology to the brink of international Table III Statistical Analysis Summary Methods used International method Aus/NZ International method FDA-FM FDA-FM Aus/NZ Nmaterials 29 36 28 LSM SPF 18.22 18.34 19.93 19.31 17.12 17.93 |SPFY – SPFX| 0.12 0.62 0.81 R 0.94 0.99 0.95
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)