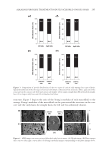
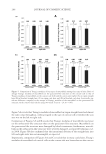
EFFECT OF ISOFERULIC ACID ON MUSHROOM TYROSINASE 237 the presence of isoferulic acid in different concentration levels (cI) is shown in Figure 1. The lag period and inhibition rate against monophenolase activity of tyrosinase (IM) increased accordingly with the increase in concentration of isoferulic acid, as shown in Figure 2. The lag period was estimated to be 1.1 min in the absence of inhibitor and almost quadrupled to 4.3 min in the presence of 0.20 mmol/L isoferulic acid. The inhibitor concentration lead- ing to 50% monophenolase activity lost (IC50) was estimated to be 0.13 mmol/L. EFFECT OF ISOFERULIC ACID CONCENTRATION ON DIPHENOLASE ACTIVITY OF TYROSINASE The inhibitory effect of isoferulic acid on the oxidation of L -DOPA via tyrosinase was also studied. As shown in Figure 3, the kinetic curves of the oxidation of L -DOPA in the pres- ence of isoferulic acid in different concentration levels indicate that there is no lag period in L -DOPA oxidation. Increasing the concentration of isoferulic acid (cI) resulted in a rapid increase of the inhibition rate against the diphenolase activity of tyrosinase (ID), reaching a fairly high inhibition rate of 73.2% when cI was 0.80 mmol/L, as shown in Figure 4. The inhibitor concentration leading to 50% diphenolase activity lost (IC50) was estimated to be 0.39 mmol/L. KINETIC PARAMETERS IN L-DOPA OXIDATION VIA TYROSINASE WITH ISOFERULIC ACID AS INHIBITOR A steady-state analysis was performed to estimate the inhibition type and the kinetic parameters of the reaction system during the oxidation of L -DOPA. Lineweaver–Burk plot for the inhibitory effect of isoferulic acid against diphenolase activity of tyrosinase was shown in Figure 5. Figure 1. Progress curves of L -tyrosine oxidation via tyrosinase with isoferulic acid as inhibitor.
JOURNAL OF COSMETIC SCIENCE 238 Under the conditions employed in the current investigation, the oxidation of L -DOPA by tyrosinase complies with a Michaelis–Menten mechanism. However, since the experiments were carried out in air-saturated aqueous solutions, the Michaelis constant (Km) and the maximum reaction rate (vm) deduced are only apparent. The effect of oxygen concentration on the kinetic parameters needs to be further investigated in subsequent studies. The result illustrated in Figure 5 shows that isoferulic acid is a competitive inhibitor, as increasing the concentration of isoferulic acid resulted in a family of lines with different slopes but sharing a common intercept on the 1/v axis. Figure 2. Effect of isoferulic acid concentration on monophenolase inhibition rate and lag time. Figure 3. Progress curves of L -DOPA oxidation via tyrosinase with isoferulic acid as inhibitor.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)