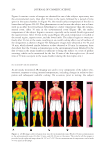
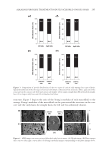
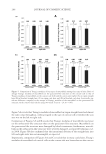
ALKALINE PEROXIDE TREATMENT INDUCES ACQUIRED UNRULY HAIR 267 side, even between the same type of cortical cells. This observation clarifi es the fact that four types of macrofi brils (on the orthocortex- or paracortex-like structure, and on the concave or convex side) are present. Another interesting fi nding is shown in Figure 5D. In comparison with Figures 5C and D, Young’s modulus of macrofi brils on the orthocortex-like structure decreased upon bleach treatments, as seen in straight hairs. Young’s modulus of macrofi brils on the orthocortex- like structure was decreased by a factor of 0.91 (= 0.88/0.97) on the concave side, whereas it was decreased by a factor of 0.84 (= 0.69/0.82) on the convex side. Comparison of Figures 5B and D reveals that the infl uence of bleach treatment on macrofi brils on the orthocortex-like structure on the concave side of curly hairs was similar to its infl u- ence in straight hairs. However, it is worth noting that macrofi brils on the orthocortex- like structure on the convex side of curly hairs was more signifi cantly infl uenced by bleach treatment than corresponding macrofi brils in straight hairs. CONCLUSION In summary, in the case of curly hairs, bleach treatments decrease the ROC. Furthermore, force curve measurements of the inner components revealed a difference in Young’s mod- ulus between macrofi brils on the concave and convex sides. Previously, it was considered that the curliness of hair is primarily infl uenced by the distributions of the two types of cortical cell. However, this study demonstrates that there are four types of macrofi brils (on the orthocortex- or paracortex-like structure, and on the concave or convex side) whose mechanical properties are different. Among these, macrofi brils on the orthocortex- like structure on the convex side of curly hairs are especially affected by bleach treatment. This could play a role in shape changes that result in unintentional unruly hair acquired by chemical bleaching with alkaline peroxide. REFERENCES (1) G. Loussouarn, A. Garcel, I. Lozano, C. Collaudin, C. Porter, S. Panhard, D. Saint-Léger, and R. Mettrie, Worldwide diversity of hair curliness: A new method of assessment. Int. J. Dermatol., 46(S1), 2–6 (2007). (2) R. D. Sinclair, Healthy hair: What is it? J. Investig. Dermatol. Symp. Proc. 12, 2–5 (2007). (3) S. Nagase, T. Shinozaki, M. Tsuchiya, H. Tsujimura, Y. Masukawa, N. Satoh, T. Itou, and K. Koike, Characteristic microstructure of curved human hair. J. Soc. Cosmet. Chem. Jpn. 43, 201–208 (2009). (4) W. G. Bryson, D. P. Harland, J. P. Caldwell, J. A. Vernon, R. J. Walls, J. L. Woods, S. Nagase, T. Itou, and K. Koike, Cortical cell types and intermediate fi lament arrangements correlate with fi ber curvature in Japanese human hair. J. Struct. Biol. 166, 46–58 (2009). (5) R. D. B Fraser and G. E. Rogers, The bilateral structure of wool cortex and its relation to crimp. Aust. J. Biol. Sci. 8, 288–299 (1955). (6) S. Thibaut, P. Barbarat, F. Leroy, and B. A. Bernard, Human hair keratin network and curvature. Int. J. Dermatol. 46(S1), 7–10 (2007). (7) M. Tate, Y. Kamath, S. B. Ruetsch, and H. D. Neigmann, Quantifi cation and prevention of hair dam- age. J. Soc. Cosmet. Chem. 44, 347–371 (1993). (8) H. Zahn, S. Hilterhaus, and A. Strussmann, Bleaching and permanent waving aspects of hair research. J. Soc. Cosmet. Chem. 37, 159–175 (1986). (9) Y. Masukawa, H. Tsujimura, H. Tanamachi, H. Narita, and G. Imokawa, Damage to human hair caused by repeated bleaching combined with daily weathering during daily life activities. Exog. Dermatol. 3, 273–281 (2004).
JOURNAL OF COSMETIC SCIENCE 268 (10) A. Kuzuhara, Analysis of structural changes in bleached keratin fi bers (black and white human hair) using Raman spectroscopy. Biopolymers 81, 506–514 (2006). (11) D. Wang, S. Fujinami, K. Nakajima, and T. Nishi, True surface topography and nanomechanical map- ping measurements on block copolymers with atomic force microscopy. Macromolecules 43, 3169–3172 (2010). (12) D. Wang, S. Fujinami, K. Nakajima, K. Niihara, S. Inukai, H. Ueki, A. Magario, T. Noguchi, M. Endo, and T. Nishi, Production of a cellular structure in carbon nanotube/natural rubber composites revealed by nanomechanical mapping. Carbon 48, 3708–3714 (2010). (13) H. Liu, S. Fujinami, D. Wang, K. Nakajima, and T. Nishi, Nanomechanical mapping on the deformed poly(ε-caprolactone). Macromolecules 44, 1779–1782 (2011). (14) H. Kitano, A. Yamamoto, M. Niwa, S. Fujinami, K. Nakajima, T. Nishi, and S. Naito, Young’s modu- lus mapping on hair cross-section by atomic force microscopy. Compos. Interfaces 16, 1–12 (2009). (15) H. Kitano, A. Yamamoto, T. Kojima, S. Yamanouchi, M. Niwa, S. Fujinami, K. Nakajima, T. Nishi, and S. Naito, Effects of chemical treatments on mechanical properties of hair internal components by atomic force microscopy. 25th IFSCC Congress Proc. (2008). (16) D. Hrdy, Quantitative hair form variation in seven populations, Am. J. Phys. Anthropol. 39, 7–17 (1973). (17) J. L. Hutter and J. Bechhoefer, Calibration of atomic force microscope tips. Rev. Sci. Instrum. 64 (7), 1868–1873 (1993). (18) S. Fujinami, H. Nukaga, K. Nakajima, and T. Nishi, Nanomechanical property analysis of polymer surfaces by atomic force microscopy. J.Surf. Sci. Soc. Jpn. 27, 530–534 (2006).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)