COSMETIC AND AMINO ACID ANALYSIS OF HAIR 289 Procedure. The clinical study was conducted according to internationally recognized Good Clinical Practice Guidelines (8). This was a randomized, single-blind, half-head study. Subjects received a baseline treatment of hair wash with a normal shampoo. Three days later, the hair was parted centrally from brow to nape of the neck by the researcher who then carried out a half-head (either left side or right side) application of the relaxers ac- cording to a randomization list. The no-lye relaxer was applied immediately after mixing. The relaxer was left on until the subject complained of scalp tingling (up to 20 min) and then it was washed off using the neutralizing shampoo. Approximately 50 strands of hair were sampled from each side treated with Product A (the lye relaxer) and from the side treated with Product B (the no-lye relaxer) of each subject. Subjects used six parameters to assess the quality of their own hair before and after relaxer treatment. These were length (cm), damage (5 = not damaged 1 = extremely damaged), straightness (4 = very straight 1 = not straight), softness (3 = very soft 1= not soft), shininess (3 = very shiny 1 = not shiny), and volume (3 = thin 1 = thick). The researcher also assessed these six parameters for each subject, together with split ends (3 = low 1 = high), dryness (4 = oily 1 = very dry), and wash-off time (min). The parameters were ranked in such a way that the higher the number the better the performance. Data were collected on the day of the treatment using questionnaires for researcher and subject self-assessments. The Wil- coxon rank sum test for nonparametric data was used to test observed differences between groups for statistical signifi cance. AMINO ACID ANALYSIS BY RP-HPLC Instrumentation. A Waters Breeze HPLC system (Waters, Millipore Corp., Milford, MA) was used for the amino acid analysis. The system included a workstation for performing pre-column hydrolysis and derivatization. Empower 3 Chromatography Data Software was used to process all data. Reagents. Acetonitrile, triethylamine, sodium acetate, glacial acetic acid, hydrochloric acid, phenylisothiocyanate (PITC), ethanol, L -α-amino-n-butyric acid (AAB), and L -cystine were obtained from Sigma-Aldrich (Johannesburg, SA). Amino acid standard H from Pierce (Prod no. 20088) was obtained from Waters (Milford, MA) through the local Sup- pliers, Separations (Johannesburg, SA). It is a quantitative mixture of 17 free amino acids, each supplied at a concentration of 2.5 μmol/ml of 0.1 N HCl, for use as a high-purity calibration standard for HPLC analysis of protein hydrolysates. It includes L -Alanine, L -Arginine, L -Aspartic acid, L -Cystine, L -Glutamic acid, L -Glycine, L -Histidine, L -Isoleucine, L -Leucine, L -Lysine, L -Methionine, L -Phenylalanine, L -Proline, L -Serine, L -Threonine, L -Tyrosine, and L -Valine. Ultrapure water was supplied by a Milli-Q purifi cation system (Millipore, Bedford, MA). Sample Preparation. Hydrolysis: Stock solutions containing 1.0 mg/ml were prepared by dissolving 10mg hair samples (in duplicate) in 10 ml of 0.1 M HCl. Volumes correspond- ing to 50 μg sample were dried under vacuum and hydrolysed with acid vapours from 200 μl of 6 M HCl at 110°C for 24 h. Derivatization: Pre-column derivatization of the amino acids with a chromophore was carried out to enable UV absorbance detection. A modifi ed Pico-Tag method (9–12) was used for derivatization (and analysis). As much as 50 μl solution of PITC in ethanol was
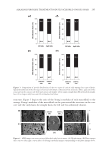
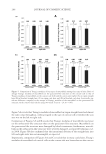
JOURNAL OF COSMETIC SCIENCE 290 added to the dry hydrolysates and the mixtures were allowed to react in the sealed vac- uum vial for 20 min at room temperature to produce phenylthiocarbamyl amino acids. The standard H was also loaded and derivatized. Analysis of samples. A Pico-Tag column (Waters, Millipore Corp., Milford, MA): C-18 bonded phase, reversed- phase mode, 3.9 × 150 mm, silica particle size 4 μm, pore size 60 Å, was used for the separations. The column temperature was controlled at 45 ± 1°C. The sample injection volume and fl ow rate were 20 μl and 1.5 ml/min, respectively. The solvent system consisted of two eluents: A, 50 mM sodium acetate aqueous buffer pH 5.5 and B, 60:40 acetonitrile:water. The program was run using a gradient of A and B with an initial 12% B and ending with 53% B after 10min. All the amino acids eluted in less than 10 min. After this, a wash-up step was programmed to 100% B so that any residual sample components would be cleaned from the column. Analytes were detected at 254 nm using a UV absorbance detector. A calibration fi le generated from analysis of the standard H was used to determine the amino acid content of the samples. Peak areas were used for quantitation. Method validation. Samples were analyzed in duplicate to obtain mean values. AAB was used as an internal standard to minimize between-runs variability. The accuracy of the method was checked by spiking samples with a standard cystine solution and determin- ing the percentage recoveries. RESULTS AND DISCUSSION CLINICAL STUDY There was no erythema or any other adverse event observed on any subject during the treatments used in the study. The relaxer wash-off times are shown in Table I. Although the mean wash-off time was longer for the no-lye relaxer, the difference (p = 0.069) was found not to be statistically signifi cant (p 0.05). Each subject assessed the quality of their own hair in terms of six parameters: length, damage, straightness, softness, shininess, and volume. The self-assessment results are summarized in Table II. Table III represents a summary of the results of the researcher assessment of nine hair quality parameters. These were the six assessed by the subjects, together with split ends, dryness, and wash-off time. Table I Wash-off Times (Min) Subject no. Product A (Lye) Product B (No-lye) p-value 1 15 15 0.690 2 8 9 3 20 20 4 9 10 5 15 17
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)