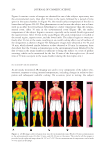
Table I Comparison of SPF in Methods(7) Parameters International 2006 Australia 1998 FDA 1999 FDA 2011 Source of UV radiation Acceptance Limits %RCEE UVAII/UVAI % RCEE defi ned in different bands λ range (nm) Erythemal Effective Radiation (%) λ range (nm) RCEE% ≤290 0.1 ≤290 0.1 290–300 1.0 – 8.0 290–300 1.0–8.0 290–310 49.0–65.0 0.01% 290 nm 290 nm to 400 nm 290–310 49.0–65.0 290–320 85.0–90.0 “red” and “blue” acceptance limits (4 nm): graph 1% energy 290 nm 290–320 85.0–90.0 290–330 91.5–95.5 ≤5% energy 400 nm 290–330 91.5–95.5 290–340 94.0–97.0 290–340 94.0–97.0 290–400 99.9–100 290–400 99.9–100 UVAII 20% UVAI 60% of the total UV irradiance to ensure that appropriate amounts of UVA radiation are included UVAII 20% UVAI 60% of the total UV irradiance to ensure that appropriate amounts of UVA radiation are included UV exposures Progression of UV dose Geometric progression of either (1.25) n or (1.12n) for the unprotected area. For the protected areas, a minimum of fi ve subsites centered on the expected SPF × MEDu shall be exposed with a geometric progression of either (1.25) n or (1.12n) Unprotected MED re-determined with a dose range of ≈ 0.6 to 1.5 provisional MEDu Geometric progression (1.25n) for the unprotected area Geometric progression (1.25) n for the unprotected area DISPARATE SPF TESTING METHODOLOGIES 299
Table I Continued Parameters International 2006 Australia 1998 FDA 1999 FDA 2011 A maximum progression of 1.12must n be used for expected SPF 25 For protected skin the dose range is multiplied by the expected SPF For the protected areas geometric series of fi ve exposure where the middle exposure is placed to yield the expected SPF plus two other exposures placed around the middle exposure For the protected areas geometric series of fi ve exposure where the middle exposure is placed to yield the expected SPF plus two other exposures placed around the middle exposure Increments between subsites no more than 1.35 According to the expected SPF () According to the expected SPF () SPF 8:0.64,×0.80,×0.90,× 1.00,×1.10,×1.25,× 1.56× SPF 8:0.64,×0.80,× 1.00× 1.25,× 1.56× ≤1.118 for SPF ≥ 25 SPF 8 to 15: 0.69,×0.83,× 0.91,×1.00,×1.09,× 1.20,×1.44× SPF 8 to 15: 0.69,× 0.83,×1.00,× 1.20,× 1.44× SPF 15: 0.76,× 0.87× 0.93,×1.00,×1.07,× 1.15,×1.32× SPF 15: 0.76,× 0.87,×1.00,× 1.15,× 1.32× Reference sunscreen formulations Reference sunscreen formulations used Expected SPF 20: P2 or P3 or P7 On each subject either: Homosalate 8% with SPF 4.47 (SD: 1.279) P3 (Padimate O 7.0% + Oxybenzone 3.0%) with SPF 16.3 (SD: 3.43) Expected SPF ≥ 20: P2 or P3 Homosalate 8% with SPF 4.47 The same reference has to be tested on every subject in the same series of at least 10 subjects P3 with SPF 15.5 Or values derived from the laboratory’s historical record on its test results JOURNAL OF COSMETIC SCIENCE 300
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)