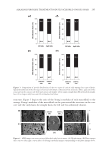
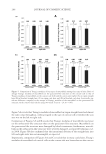
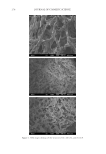
J. Cosmet. Sci., 64, 273–286 ( July/August 2013) 273 Lauroyl/Palmitoyl glycol chitosan gels enhance skin delivery of magnesium ascorbyl phosphate PO-CHUN WANG, YAN-LING HUANG, SHENG-SHU HOU, CHEN-HSI CHOU, and JUI-CHEN TSAI Institute of Clinical Pharmacy and Pharmaceutical Sciences, College of Medicine, National Cheng Kung University, Taiwan (P.C.W., Y.L.H., C.H.C., J.C.T.), Department of Chemical Engineering, College of Engineering, National Cheng Kung University, Taiwan (S.S.H.). Accepted for publication October 4, 2012. Synopsis Palmitoyl glycol chitosan (GCP) hydrogel has been reported as erodible controlled-release systems for the delivery of both hydrophilic and hydrophobic molecules. In this study we prepared lauroyl/palmitoyl glycol chitosan (GCL/GCP) in gel form and evaluated their application for skin delivery of the hydrophilic com- pound, magnesium ascorbyl phosphate (MAP), which is widely used in cosmetic formulations. Release of MAP from the polymer gels was signifi cantly decreased with increasing concentration of GCL/GCP in the formulations in comparison with glycol chitosan (GC). In both aqueous and 10% ethanol vehicles, MAP fl ux was increased 1.58- to 3.96-fold of 1% GC from 1% GCL/GCP. Increase in MAP fl ux was correlated to the increase in GCL/GCP concentration prepared in 10% ethanol vehicle. GCL/GCP, in either water or 10% ethanol vehicles, increased the skin penetration and skin deposition of MAP in comparison with GC, hy- droxypropylmethylcellulose, and carbopol, while sustaining its release from the polymer gels. Both the en- hancement in skin penetration/deposition and sustained release of MAP were depended on polymer concentration. Also, with increase in polymer concentration, epidermal to dermal drug deposition ratio tended to increase, which will be benefi cial to its activity in the epidermis, such as inhibition of tyrosinase and protection from UV damage. These data suggested both GCL and GCP can be applied as delivery vehi- cles to improve percutaneous absorption of MAP. INTRODUCTION Magnesium ascorbyl phosphate is a derivative of L -ascorbic acid (Vitamin C) that is known to be stable in the light, air or heat. After entering the human or animal body, it is converted into L -ascorbic acid through the enzymolysis of phosphatase, and maintains the same physiological function and biological effi cacy as L -ascorbic acid. MAP is a key constituent of skin moisturizer intended for effective inhibition of tyrosinase activity in skin and facilitation of collagen synthesis. MAP also resists the damage caused on the Address all correspondence to Jui-Chen Tsai at jctsai@mail.ncku.edu.tw.
JOURNAL OF COSMETIC SCIENCE 274 skin due to the UV light, smog, and other environmental effects and hence has been widely used in cosmetics (1–3). In addition, it is known that MAP may stimulate growth of human dermal fi broblast cells (4,5). It is also shown that MAP stimulates growth of dermal papilla cells in vitro and early conversion from telogen phase to anagen phase in mice (6). Furthermore, MAP treatment resulted in signifi cant elongation of hair shafts in isolated hair follicles. It has been suggested that the responsible mechanisms include its proliferative and antiapoptotic effects on dermal papilla cells (6). Chitosan, a polysaccharide comprising copolymers of glucosamine and N-acetylglucosamine, is derived by deacetylation of chitin, the second abundant polysaccharide present in nature (7). It has been regarded as biocompatible, biodegradable, nontoxic, and non- immunogenic, and is an interesting biomaterial because of its ability as a cosmetic carry- ing materials and ease of modifi cation (8). Despite its superiority as a biomaterial, chitosan is only soluble in acidic solutions, which limits its application (9). Water-soluble glycol chitosan (GC) is derived from chitosan, and is emerging as novel carriers of drugs (10). To improve its pharmaceutical acceptability, GC was modifi ed with hydrophobic groups (fatty acids) to form a noncovalently cross-linked amphiphilic hydrogels (11,12). GCP hydrogel has been shown as erodible controlled-release systems for the delivery of both hydrophilic macromolecules and hydrophobic drugs (13,14). In this work, we de- scribe the use of lauroyl/palmitoyl glycol chitosan (GCL/GCP) in the form of gel as sup- porting material for skin absorption of hydrophilic molecules using MAP as model compound. The use of an amphiphilic polymer (GCL/GCP) to prepare gels may add the potential advantage of improving skin delivery of MAP for cosmetic applications. EXPERIMENT MATERIALS GC (Mw 128 kDa degree of polymerization ≥400), palmitic acid N-hydroxysuccinimide (PNS), lauric acid N-hydroxysuccinimide (LNS), hydroxypropylmethylcellulose (HPMC), L -Ascorbic acid 2-phosphate sesquimagnesium salt hydrate, phosphate buffered saline (PBS), and sodium bicarbonate were purchased from Sigma chemical Co. (St. Louis, MO). Carbopol 940 was obtained from ACROS Organics (Morris Plains, NJ). Chloral hydrate was purchased from Tokyo Chemical Inc. (Tokyo, Japan). Absolute ethanol was purchased from Panreac Quimica (Barcelona, Spain). Acetone and diethyl ether were obtained from Merck Co. (Darmstadt, Germany). Dialysis tubing of molecular weight cut-off (MWCO) 12–14 kDa was obtained from Millipore Co. (Billerica, MA). SYNTHESIS OF GCL/GCP The synthesis of GCL/GCP was carried out according to the methods described in Noble et al. (11) and Cerchiara et al. (12). Briefl y, GC (100 mg) was dissolved in Milli-Q water (20 ml), to which sodium bicarbonate (76 mg) and absolute ethanol (10 ml) were added. A solution of LNS (133 mg) or PNS (158 mg) in absolute ethanol (10 ml) was added dropwise to the alkaline solution of GC over 1-h period. After 72 h of stirring protected from light, acetone (20 ml) was added to the reaction mixture and the resulting solution
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)