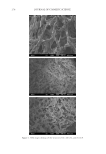
GCL/GCP GELS ENHANCE SKIN DELIVERY OF MAP 275 was evaporated under reduced pressure at 45°C to remove organic solvents. The residual mixture was extracted with diethyl ether (40 ml) and subjected to exhaustive dialysis within dialysis tubing for 24 h with six changes of water, and were centrifuged at 4,500 rpm for 10 min at 4°C and then freeze-dried. PHYSICOCHEMICAL CHARACTERIZATION OF GCL/GCP Scanning electron microscopy (SEM) images of the specimens were recorded using a JSM- 6700F microscope. FTIR spectra of the specimens were obtained with a Nicolet Magna 560 Spectrometer. Samples of GCL/GCP were ground into a powder form, mixed with KBr (1:100), and pressed into a disk and analyzed. Molecular weights of GCL/GCP were deter- mined by gel permeation chromatography (GPC) run at 40°C in 0.05M NaNO3–NaN3 water (15) (at a fl ow rate of 0.5 ml/min) on a Waters 510 HPLC pump with an Ultrahydro- gel™500 column using a RI-detector (Perkin Elmer, Norwalk, CT). The injection volume was 150 μl of a 0.48% w/w polymer in 10% ethanol. Molecular weight calculations were based on a linear calibration curve obtained using molecular weight standards Pullulan (Shodex standard WAT034207). Viscosity of 1–3% polymer gels prepared in Milli-Q water and 10% ethanol was analyzed by VISCO Star-R (Fungilab, Spain) at 37°C for 30 min. 1 H NUCLEAR MAGNETIC RESONANCE (NMR) SPECTRA STUDY 1 H nuclear magnetic resonance (NMR) correlation spectroscopy experiments (Bruker AV- 500 NMR spectrometer, Darmstadt, Germany) were performed on solutions of GC and GCL/GCP in CF3COOD to assign non-exchangeable coupled protons (11). Levels of lauroylation/palmitoylation in mol% of GC were estimated by the ratio given below. GCL/GCP lauroyl/palmitoyl methyl proton (δ 0.85–0.93 ppm)/acetyl proton (δ 1.83 ppm) IN VITRO RELEASE/SKIN PERMEATION APPARATUS Both release and skin permeation experiments were performed using vertical type fl ow- through diffusion cells (Laboratory Glass Apparatus, Berkeley, CA) with an effective diffu- sion area of 1 cm2 and a receiver volume of 3.6 ml, and maintained at a constant temperature with a 37°C-circulating water bath. Buffers were pumped through the receiver compart- ment at a fl ow rate of 3–4 ml/h with a peristaltic pump (Ismatec, Glattbrug-Zürich, Swit- zerland), and the fl uid in the receptor phase was stirred continuously at 700 rpm. PREPARATION OF MAP-CONTAINING GCL/GCP GELS At least three batches of synthesized polymers were mixed for the preparation of MAP formulations. Appropriate amounts of polymers were mixed with 3% MAP solution in Milli-Q water or 10% ethanol, and equilibrated in an orbital shaker overnight to form 1–3% gel.
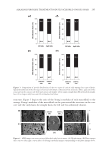
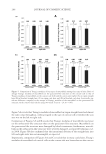
JOURNAL OF COSMETIC SCIENCE 276 IN VITRO RELEASE STUDIES The release membrane (Spectra/por® MWCO 1,000 Dalton) was clamped between the donor and receptor phases of the diffusion cells with the receptor phase fi lled with normal saline (0.9% sodium chloride), which has been adjusted to pH 6.0 by acetic acid. 500 μl of 3% MAP gels were dosed onto the membrane surface. Receiver solutions were col- lected every hour for 6 h and analyzed by HPLC. IN VITRO SKIN PERMEATION STUDIES Full-thickness dorsal skin from ICR nude mice (Nar1: ICR-Foxn1nu, National Labora- tory Animal Center, Taiwan) was used for all the permeation experiments. The skins were mounted between the donor and receptor phase of the diffusion cells with the receptor phase fi lled with PBS having pH 7.4. As much as 500 μl of MAP gels were applied to the skin surface, and then occluded with parafi lm. The receiver solution was collected at 1-h intervals for 6 h. At the end of 6 h, the skin segment was dismounted from the diffusion cells, rinsed with Milli-Q water for three times, and gently dried with Kimwipes® to remove the residual gels. The skin was placed on glass disc and heat-separated into epi- dermis and dermis at 60°C water bath for 1.5 min. Both the separated epidermis and dermis were weighed and homogenized in 1 ml phosphate buffer (pH 5.0) using a Poly- tron PT210 homogenizer. The homogenates were centrifuged at 14,500 rpm for 20 min. MAP concentrations in the supernatants and receiver fractions were determined by HPLC. HPLC ASSAY OF MAP The HPLC system consists of a Waters 510 pump (Milford, MA), an autosampler (Waters 717), a UV detector (Waters 486), and a workstation (SISC 32, Taipei, Taiwan). The column was a C18 reversed-phase column (Purospher® star, 4.6 × 150 mm, 5 μm), eluted with a mobile phase of acetonitrile: 50 mM NaH2PO4 with 2 mM cet- yltrimethylammonium bromide (adjusted with H3PO4 to pH 5) (5:6 v/v) at a fl ow rate of 0.7 ml/min. The detection wavelength was set at 255 nm, and MAP was de- tected at a retention time of 8 min. The assay was linear in the concentration range of 0.2–20.0 μg/ml. The inter- and intra-day assay accuracy (% error) and precision (% CV) were between −2.0% and 9.0%. No interference from the GCL/GCP gels or skin tissue was observed. DATA ANALYSIS Both the release and skin permeation experiments for each preparation were repeated three to six times and data were expressed as the mean value ± S.D. Release rate and skin fl ux were calculated from slope of the linear part of the cumulative amount of MAP re- leased/penetrated vs. time curve. Statistical analyses were performed using one-way ANOVA test. A p value 0.05 was considered statistically signifi cant.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)