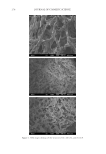
ENVIRONMENTAL PARAMETERS ON SWEAT GLAND ACTIVITY 247 on sweating was investigated by giving the subjects 250 ml of water at t = 20 min in the sauna (water was consumed within 5 min). RHEOLOGICAL ANALYSIS AND PREPARATION OF REPLICAS To obtain replicas of the skin-surface structure, including sweat bead topography, we used Silfl o impression resin in combination with supplied thinner and catalyst (CuDerm, Dallas, TX). The preparation consisted of (i) Adding 10 drops of thinner to 4.0 g of resin, mixing thoroughly, and allowing 20 min for equilibration. (ii) Five drops of catalyst were subsequently added to the resin/thinner mixture, followed by 10 s of meticulous mixing, and ensuing application to the skin or DMA geometry. Because the resin hardens via a time-dependent, condensation curing process, where the hydroxyl-terminated silicone chains are catalyzed into a crosslinked, elastomeric network, the kinetics and rheology of the curing process were studied prior to in vivo testing. A DMTA Mark IV Dynamic Me- chanical Analyzer (TA Instruments, New Castle, DE) was used to approximate the gel point (i.e., E′ = E″) of the curing resin (14). Aliquots of the catalyzed resin were loaded into a compression fi xture (17-mm-diameter stainless steel plates) and dynamic time sweep experiments (ω = 1 Hz, γ = 0.5%, gap = 1 mm, 25.7°C, n = 6) were executed to follow the changes in viscoelasticity as a function of time. To simulate the presence of a bead of sweat in contact with the curing resin, a 20 μl droplet of ρ = 18.2 MΩ·cm puri- fi ed water (Millipore, Burlington, MA) was added to the surface of the loaded resin prior to adjusting the DMA geometry to the 1-mm testing gap and starting the test. During the in vivo perspiration tests, silicone impressions were only taken during Test 1. In this particular test, we used Procedure 1 (described in the section above) in which case approximately 10 drops of thinner is added to the impression material at t = 10 min in the sauna. This mixture is allowed to sit until t = 30 min, when 5 drops of catalyst were added. The resulting mixture is thoroughly, but gently, mixed prior to application avoiding the formation of air bubbles (note that mixing was carried out in the environmentally con- trolled room, not the sauna). Four skin replica rings were placed on the upper part of the inner forearms of the subjects for the last 5 min they spent in the sauna. Also, the day prior to experiments, all subjects were thoroughly shaved in the inner former area so that hair fi bers would not interfere with the replica measurements of sweat protrusions. Subjects did not use any type of cosmetic or pharmaceutical products on their inner forearm region for at least 1 day prior to the examination (In fact, in almost all cases, subjects reported never treating their skin with products.). Prior to the tests, the forearm of each subject was gen- tly washed with Dove soap (Dove beauty bar Unilever, Trumbull, CT). Image analysis methods were used in combination with skin replica analysis to quantify the number of active sweat glands per given area. This technique is largely based on a published report by Keyhani et al. (13). Image processing and analysis were carried out using Adobe Photoshop CS5 (Adobe Systems Inc., San Jose, CA) and ImageJ (http://rsbweb.nih.gov/ij/). IR THERMAL IMAGING We conducted thermal imaging measurements with a FLIR Systems camera (Model P620 FLIR Systems Inc., Wilsonville, OR). The thermal imaging camera was mounted on a tripod
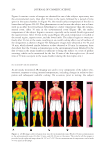
JOURNAL OF COSMETIC SCIENCE 248 at a fi xed distance from the subject to obtain a full-body image. All dimensions were kept constant throughout the experiments. The image data consist of a two-dimensional image grid with temperature measurements plotted on a third axis using a color image scale. FLUX DENSITY MEASUREMENTS To gain insight into the impact of a subject’s recent environmental history on the quality of the fi nal 30 min acclimatization process (i.e., equilibration phase before entering the hot box), a new method was applied in vivo to two experienced subjects. Vapor fl ux measure- ments were performed on the two male subjects after 15 min pre-equilibration in a cool (6.3°C, 66% RH) or warm (24°C, 28% RH) environment. Flux density measurements were commenced during the fi nal acclimatization stage (30.5°C, 36.5% RH) using an AquaFlux AF200 (Biox Systems, London, UK) evaporimeter confi gured with a Tefl on 7 mm orifi ce measurement cap. The AF200 was used to examine variations in the vapor fl ux density as a function of equilibration time. For each subject, three zones of the middle of the left volar forearm were screened at 0, 30, and 60 min of the fi nal acclimatization process. The device was transferred rapidly from park (idle), or from zone to zone, to reduce the impact of ambient humidity on the skin surface water loss results. The maximum time for each zone measurement was 80 s and the target precision for the evaluation of the equilib- rium fl ux density was ≤0.075 gm−2h−1 standard deviations, which is calculated from the running average of 10 consecutive fl ux readings. Two-sample Student’s t-tests (α = 0.10, two-tailed), assuming equal variances, were performed on the fl ux density results to test the null hypotheses (H0: μ1 − μ2 ≠ 0) that (i) environmental pre-equilibration time and condi- tions have no infl uence on a single subject’s fl ux density response (ii) in addition, when compared at the same pre-equilibration time and environmental conditions, the fl ux den- sity response between subjects is not statistically signifi cant (XLSTAT, Addinsoft, NY). RESULTS AND DISCUSSION We used several techniques to monitor the amount of perspiration by test participants. A skin replica technique was used following a previously published procedure (13). To gain further insight into the gelation of the replica material, we carried out rheological mea- surements using DMA. In turn, a method similar to the FDA monograph was used to measure axillae sweating using absorbent pads (15). We also used thermal IR imaging to monitor overall skin-surface temperature and the anatomical temperature distribution. In all of these tests, subjects were fi rst acclimated in an environmentally controlled room (27°C, 50% RH) for 30 min and then placed in a similarly controlled sauna chamber— specifi c temperature and humidity settings are provided in the following text. In addi- tion, we monitored variations in fl ux density of water vapor diffusing through the skin barrier either in the form of TEWL and/or insensible perspiration. RHEOLOGY OF SILFLO FORMULATION Figure 1 shows the average (n = 6) curing profi le of the Silfl o replica formulation at ambi- ent conditions as a function of time. The error bars express the standard deviation of
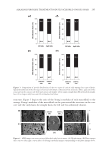
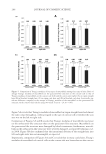
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)