GCL/GCP GELS ENHANCE SKIN DELIVERY OF MAP 285 GCL/GCP can also be applied for skin delivery of MAP in the form of gels. GCL/GCP gels prepared in either water or 10% ethanol vehicles increased the skin penetration and skin deposition of MAP in comparison with GC, HPMC, and carbopol, while sustained its release from the polymer gels. The reduction in MAP release was attributed to its viscosity resulting from cross-link structure, whereas increase in skin penetration and deposition may be result of polymer interaction with stratum corneum. Both the en- hancement in skin penetration/deposition and sustained release of MAP were dependent on polymer concentration. Also, with increase in polymer concentration, epidermal to dermal MAP deposition ratio tended to increase, which will be benefi cial to its activity in the epidermis, such as inhibition of tyrosinase and protection from UV damage. The data suggested both GCL and GCP gels can be applied as skin delivery vehicles to im- prove percutaneous absorption of MAP for cosmetic purposes. ACKNOWLEDGMENTS This work was supported by grants from the National Science Council of Taiwan (NSC 98-2320-B-006-015). REFERENCES (1) S. R. Pinnell, Cutaneous photodamage, oxidative stress, and topical antioxidant protection, J. Am. Acad. Dermatol., 48, 1–19 (2003). (2) M. P. Lupo, Antioxidants and vitamins in cosmetics, Clin. Dermatol., 19, 467–473 (2001). (3) P. M. Campos, F. B. de Camargo Júnior, J. P. de Andrade, L. R. Gaspar, Effi cacy of cosmetic formulations containing dispersion of liposome with magnesium ascorbyl phosphate, alpha-lipoic acid and kinetin, Photochem. Photobiol., 88, 748–752 (2012). (4) R. Hata and H. Senoo, L-ascorbic acid 2-phosphate stimulates collagen accumulation, cell proliferation, and formation of a three-dimensional tissuelike substance by skin fi broblasts, J. Cell. Physiol., 138, 8–16 (1989). (5) S. Takamizawa, Y. Maehata, K. Imai, H. Senoo, S. Sato, and R. Hata, Effects of ascorbic acid and ascor- bic acid 2-phosphate, a long-acting vitamin C derivative, on the proliferation and differentiation of human osteoblast-like cells, Cell Biol. Int., 28, 255–265 (2004). (6) Y. K. Sung, S. Y. Hwang, S. Y. Cha, S. R. Kim, S. Y. Park, M. K. Kim, and J. C. Kim, The hair growth promoting effect of ascorbic acid 2-phosphate, a long-acting vitamin C derivative, J. Dermatol. Sci., 41, 150–152 (2006). (7) Y. Xu, Y. Du, R. Huang, and L. Gao, Preparation and modifi cation of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier, Biomaterials, 24, 5015–5022 (2003). (8) S. Hirano, Chitin and chitosan as novel biotechnological materials, Polym. Int., 48, 732–734 (1999). (9) D. G. Kim, Y. I. Jeong, C. Choi, S. H. Roh, S. K. Kang, M. K. Jang, and J. W. Nah, Retinol-encapsulated low molecular water-soluble chitosan nanoparticles, Int. J. Pharm., 319, 130–138 (2006). (10) A. Trapani, J. Sitterberg, U. Bakowsky, and T. Kissel, The potential of glycol chitosan nanoparticles as carrier for low water soluble drugs, Int. J. Pharm., 375, 97–106 (2009). (11) L. Noble, A. I. Gray, L. Sadiq, and I. F. Uchegbu, A non-covalently cross-linked chitosan based hydro- gel, Int. J. Pharm., 192, 173–182 (1999). (12) T. Cerchiara, B. Luppi, F. Bigucci, I. Orienti, and V. Zecchi, Physically cross-linked chitosan hydrogels as topical vehicles for hydrophilic drug, J. Pharm. Pharmacol., 54, 1453–1459 (2002). (13) L. Martin, C. G.. Wilson, F. Koosha, L. Tetley, A. I. Gray, S. Senel and I. F. Uchegbu, The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels, J. Control. Release, 80, 87–100 (2002). (14) L. Martin, C. G. Wilson, F. Koosha, and I. F. Uchegbu, Sustained buccal delivery of the hydrophobic drug denbufylline using physically cross-linked palmitoyl glycol chitosan hydrogels, Eur. J. Pharm. Biopharm., 55, 35–45 (2003).
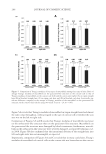
JOURNAL OF COSMETIC SCIENCE 286 (15) S. M. Dutczak, C. R. Tanardi, M. Wessling, and D. Stamatialis, Chemistry in a spinneret to fabricate hollow fi bers for organic solvent fi ltration, Sep. Pur. Tech., 86, 183–189 (2012). (16) T. Dey, Properties of vinyl ester resins containing methacrylated fatty acid comonomer: the effect of fatty acid chain length, Polym. Int., 56, 853–859 (2007). (17) T. R. Raj Singh, A. D. Woolfson, and R. F. Donnelly, Investigation of solute permeation across hydro- gels composed of poly(methyl vinyl ether-co-maleic acid) and poly(ethylene glycol), J. Pharm. Pharma- col., 62, 829–837 (2010). (18) A. Zlatanic ´, C. Lava, W. Zhang, and Z. S. Petrovic ´, Effect of structure on properties of polyols and poly- urethanes based on different vegetable oils, J. Polym. Sci. Pol. Phys., 42, 809–819 (2004). (19) A. H. Vesterinen, J. Rich, and J. Seppala, Synthesis and solution rheology of poly[(stearyl methacrylate)- stat-([2-(methacryloyloxy)ethyl] trimethyl ammonium iodide)], J. Colloid. Interface. Sci., 351, 478–484 (2010). (20) V. Sedelkin, G. Denisova, A. Surkova, L. Ramazaeva, and O. Pachina, Rheological properties of spin- ning solutions for cellulose acetate ultrafi ltration membranes, Fibre Chemistry, 39, 16–19 (2007). (21) S. Uspenskii, G. Vikhoreva, A. Sonina, and L. Gal’braikh, Properties of acetic-acid alcohol-containing solutions of chitosan, Fibre Chemistry, 42, 88–91 (2010). (22) L. W. Cheong, P. W. Heng, and L. F. Wong, Relationship between polymer viscosity and drug release from a matrix system, Pharm. Res., 9, 1510–1514 (1992). (23) G. Csóka, S. Marton, R. Zelko, N. Otomo, and I. Antal, Application of sucrose fatty acid esters in transdermal therapeutic systems, Eur. J. Pharm. Biopharm., 65, 233–237 (2007). (24) A. C. Williams and B. W. Barry, Penetration enhancers, Adv. Drug Deliv. Rev., 56, 603–618 (2004). Supplementary Figure 1. Supplementary Figure 2.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)