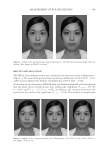
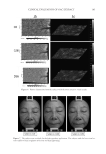
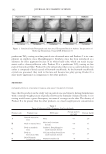
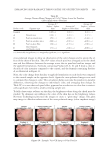
THE WHITENING AND ANTI-WRINKLE EFFECT OF PONEGRANATE 157 determines skin color. Melanin is a complex polymer derived from the amino acid tyro- sine and melanin biosynthesis, and it is regulated by melanogenic proteins, tyrosinase, tyrosinase-related protein (TRP)-1, and TRP-2 35,36). Tyrosinase activity is important for the control of melanogenesis, because it is the catalyst of the rate-limiting reaction of the melanogenic pathway. Thus, inhibition of tyrosinase activity is the most important approach to achieve skin whiteness 36,37). On the other hand, several other approaches have been used to suppress melanin synthesis, including inhibition of tyrosinase mRNA expression, aberration of tyrosinase glycosylation and maturation, acceleration of tyrosi- nase degradation, interference with melanosome maturation and transfer, inhibition of infl ammation-induced melanogenic responses, and acceleration of skin turnover. Although our data showed that PCS signifi cantly inhibits melanin synthesis and does not affect tyrosinase activity, further studies are required to examine the underlying mechanism of PCS on melanin synthesis. Last, to confi rm the protective effects of PCS on photoaging, a histomorphometrical anal- ysis was performed. In UVB-exposed mice, noticeable increases in the number of infl am- matory cells infi ltrating the dermis, abnormal collagen depositions, and formation of microfolds on the surface of the epithelial lining were observed. However, signifi cant decreases in UVB-induced histopathological dermal sclerosis and infl ammatory signs were observed in 1, 2 and 0.5 ml/kg PCS-treated hairless mice compared with UVB con- trol mice. These results indicate that PCS exerts protective effects against UVB-induced photoaging, at least under the conditions evaluated in this histopathological analysis. Acute exposure to UV irradiation causes migration of infl ammatory cells, especially the fi rst line of defense cells, neutrophils (38). This infl ammation is involved in accumulated damage to the skin induced by skin cancer and premature skin aging (39,40). In addi- tion, chronic photoaging induces marked hyperplasia of epidermal cells with hyperkera- tosis, leading to wrinkle formation (26,41) with MMP-mediated dermal sclerosis (26,33). Collectively, these results suggest that PCS, as an ingredient of functional cosmetics, could exert adequate antiwrinkle and whitening benefi ts. These effects are associated with enhanced hyaluronan synthesis, as well as suppressed elastase, collagenase, MMP-1, and tyrosinase activities and melanin production. In addition, UVB-induced histopatho- logical dermal sclerosis and infl ammatory signs were signifi cantly decreased in PCS- treated hairless mice compared with UVB-exposed mice. Therefore, these results suggest that PCS may serve as a predictable functional ingredient. ACKNOWLEDGMENT This work was supported by a National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIP No. 2011-0030124). REFERENCES (1) E. Cevenini, L. Invidia, F. Lescai, S. Salvioli, P. Tieri, G. Castellani, and C. Franceschi, Human models of aging and longevity, Expert. Opin. Biol. Ther., 8, 1393–1405 (2008). (2) B. A. Gilchrest, Skin aging and photoaging, Dermatol. Nurs., 2, 79–82 (1990). (3) M. S. Kim, Y. K. Kim, K. H. Cho, and J. H. Chung, Regulation of type I procollagen and MMP-1 expression after single or repeated exposure to infrared radiation in human skin, Mech. Ageing Dev., 127, 875–882 (2006).
JOURNAL OF COSMETIC SCIENCE 158 (4) N. Buechner, P. Schroeder, S. Jakob, K. Kunze, T. Maresch, C. Calles, J. Krutmann, and J. Haendeler, Changes of MMP-1 and collagen type Ialpha1 by UVA, UVB and IRA are differentially regulated by Trx-1, Exp. Gerontol., 43, 633–637 (2008). (5) A. Honda, R. Abe, T. Makino, O. Norisugi, Y. Fujita, H. Watanabe, J. Nishihira, Y. Iwakura, S. Yamagishi, H. Shimizu, and T. Shimizu, Interleukin-1 beta and macrophage migration inhibitory factor (MIF) in dermal fi broblasts mediate UVA-induced matrix metalloproteinase-1 expression, J. Dermatol. Sci., 49, 63–72 (2008). (6) E. Cho, E. Cho, S. Choi, K. Park, S. Kim, Y. Jeong, R. Center, C. Ku, R. Center, and B. Ha, Screening of anti-wrinkle resource from herbal medicinal extracts and stability test of its cosmetic products, Korean J. Medicinal Crop Sci., 19, 126–135 (2011). (7) F. Afaq, M. A. Zaid, N. Khan, M. Dreher, and H. Mukhtar, Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin, Exp. Dermatol., 18, 553–561 (2009). (8) M. Z. Campanini, F. A. Pinho-Ribeiro, A. L. Ivan, V. S. Ferreira, F. M. Vilela, F. T. Vicentini, R. M. Martinez, A. C. Zarpelon, M. J. Fonseca, T. J. Faria, M. M. Baracat, W. A. Verri, Jr., S. R. Georgetti, and R. Casagrande, Effi cacy of topical formulations containing Pimenta pseudocaryophyllus extract against UVB-induced oxidative stress and infl ammation in hairless mice, J. Photochem. Photobiol. B., 127, 153–160 (2013). (9) M. Yoshimura, Y. Watanabe, K. Kasai, J. Yamakoshi, and T. Koga, Inhibitory effect of an ellagic acid- rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation, Biosci. Biotechnol. Biochem., 69, 2368–2373 (2005). (10) F. Afaq, M. Saleem, C. G. Krueger, J. D. Reed, and H. Mukhtar, Anthocyanin- and hydrolyzable tannin- rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumori- genesis in CD-1 mice, Int. J. Cancer, 113, 423–433 (2005). (11) N. M. Moneam, A. S. el Sharaky, and M. M. Badreldin, Oestrogen content of pomegranate seeds, J. Chromatogr., 438, 438–442 (1988). (12) M. Aviram and M. Rosenblat, Pomegranate protection against cardiovascular diseases, Evid. Based Com- plement. Alternat. Med., 2012, 382763 (2012). (13) L. S. Adams, N. P. Seeram, B. B. Aggarwal, Y. Takada, D. Sand, and D. Heber, Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress infl ammatory cell signaling in colon cancer cells, J. Agric. Food Chem., 54, 980–985 (2006). (14) M. R. Sartippour, N. P. Seeram, J. Y. Rao, A. Moro, D. M. Harris, S. M. Henning, A. Firouzi, M. B. Rettig, W. J. Aronson, A. J. Pantuck, and D. Heber, Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo, Int. J. Oncol., 32, 475–480 (2008). (15) M. Aviram, M. Rosenblat, D. Gaitini, S. Nitecki, A. Hoffman, L. Dornfeld, N. Volkova, D. Presser, J. Attias, H. Liker, and T. Hayek, Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation, Clin. Nutr., 23, 423–433 (2004). (16) S. A. Ropacki, S. M. Patel, and R. E. Hartman, Pomegranate supplementation protects against memory dysfunction after heart surgery: A pilot study, Evid. Based Complement. Alternat. Med., 2013, 932401 (2013). (17) F. Deeba, M. M. Alam, M. R. Islam, A. Matin, R. Khan, and N. N. Ava, Laparoscopic fi ndings of sub- fertile women attended in a tertiary level care centre, Mymensingh Med. J., 22, 478–482 (2013). (18) O. Orgil, E. Schwartz, L. Baruch, I. Matityahu, J. Mahajna, and R. Amir, The antioxidative and anti- proliferative potential of non-edible organs of the pomegranate fruit and tree, LWT—Food Sci. Technol., 58, 571–577 (2014). (19) N. Khan, D. N. Syed, H. C. Pal, H. Mukhtar, and F. Afaq, Pomegranate fruit extract inhibits UVB- induced infl ammation and proliferation by modulating NF-kappaB and MAPK signaling pathways in mouse skin, Photochem. Photobiol., 88, 1126–1134 (2012). (20) J. George, M. Singh, A. K. Srivastava, K. Bhui, and Y. Shukla, Synergistic growth inhibition of mouse skin tumors by pomegranate fruit extract and diallyl sulfi de: Evidence for inhibition of activated MAPKs/NF-kappaB and reduced cell proliferation, Food Chem. Toxicol., 49, 1511–1520 (2011). (21) P. Jiang, X. Li, C. B. Thompson, Z. Huang, F. Araiza, R. Osgood, G. Wei, M. Feldmann, G. I. Frost, and H. M. Shepard, Effective targeting of the tumor microenvironment for cancer therapy, Anticancer Res., 32, 1203–1212 (2012). (22) J. Heo, J. Park, S. An, J. Lee, C. Yun, H. Shin, T. Kwon, and S. Lee, Anti-oxidant and anti-tumor ac- tivities of crude extracts by Gastrodia elata Blume, Korean. J. Food Preserv., 13, 83–87 (2006). (23) Y. Masamoto, H. Ando, Y. Murata, Y. Shimoishi, M. Tada, and K. Takahata, Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L, Biosci. Biotechnol. Biochem., 67, 631–634 (2003).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)