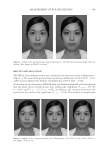
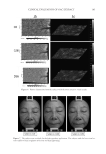
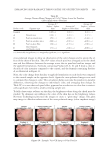
CLINICAL EVALUATION OF GAC EXTRACT 181 Safety and irritation. The product’s safety was assessed for potential adverse events. The acute skin tolerance of the cosmetic product was evaluated on 22 adult subjects via ap- plication under an occlusive patch over a 24- or 48-h period 2 samples of 30 ml or 30 g were used for each product. One product was assessed using “occlusive” or “semiocclusive” patch test types. The clinical examination of the scapular area was under dermatological control. Reactions were defi ned using the following criteria: • Erythema • Edema • Cutaneous dryness • Vesicles Table I shows the acute cutaneous tolerance of the product in 22 healthy subjects during a 48 h occlusive single patch test. Inclusion and exclusion criteria were assessed using the Mean Irritation Index (MII) (22–24). MII = Total cutaneous reactions score (erythema ± edema)/Number of subjects Table I Irritation Evaluation MII Product classifi cation MII ≤ 0.20 Non irritating 0.20 ≤ MII 0.50 Slightly irritating 0.50 ≤ MII 1 Moderately irritating MII ≥ 1 Irritating MII product classifi cation involves the following parameters: nonirritating (MII ≤ 0.20), slightly irritating (0.20 ≤ MII 0.50), moderately irritating (0.50 ≤ MII 1), and irri- tating (MII ≥ 1). No irritation (MII values of 0.00). Statistical analysis. Data are expressed as the mean ± standard deviation (S.D.) of three replicates. One-way analysis of variance (ANOVA) and Duncan’s new multiple range test were used to determine differences between means. The p values 0.05 were regarded as signifi cant. RESULTS AND DISCUSSION IN VITRO BIOLOGICAL TEST Antioxidant activities. The antioxidant activity of Gac extract was measured and compared with those of vitamins C and E. Gac extract exhibited the highest antioxidant activity in the DPPH, ABTS, and FRAP assays, as shown in Figure 4.
JOURNAL OF COSMETIC SCIENCE 182 Gac extract thus has great potential as an antioxidant and could be further developed into an antiwrinkle (11) and whitening cream. The antioxidant properties of Gac extract are at- tributable to its carotenoid and beta-carotene content (25). Using the DPPH method, the antioxidant activity of Gac (41.25 ± 0.34 mg TEAC/ml extract) was similar to that of vitamin C (41.084 ± 0.277 mg TEAC/ml extract), but 5.85-fold greater than that of vita- min E (7.045 ± 0.427 mg TEAC/ml extract). Using the ABTS method, the antioxidant activity of Gac extract (47.70 ± 0.18 mg TEAC/ml extract) was 1.27-fold greater than that of vitamin C (37.473 ± 0.136 mg TEAC/ml extract) and 11.46-fold greater than that of vitamin E (4.16 ± 1.385 mg TEAC/ml extract). Using the FRAP method, the antioxidant activity of Gac extract (105.03±2.326 mg TEAC/ml extract) was 2.87-fold greater than that of vitamin C (36.514 ± 1.244 mg TEAC/ml extract) and 2.28-fold greater than that of vitamin E (45.869 ± 1.522 mg TEAC/ml extract). However, these activities may be partly augmented by other constituents in Gac extract, e.g., phenolic compounds. Tyrosinase inhibitory activity. Tyrosinase plays an important role in the biosynthesis of mel- anin from tyrosine. Kojic acid is used in tyrosinase inhibitory activity and storage stabil- ity studies (26). Tyrosinase inhibitory activity was used to assess the whitening effect of Gac extract. The production of melanin can be decreased by inhibiting the enzyme ty- rosinase (18). Tyrosinase activity was inhibited by 62.83 ± 1.99% by Gac extract, more than the acceptable value (50%) for creams that produce a whitening effect. As shown in Figure 5, Gac extract had 1.51-fold greater tyrosinase inhibitory activity than vitamin C Figure 4. Antioxidant activity of Gac extract, vitamin C, and vitamin E measured as mg TEAC/ml extract. Figure 5. Tyrosinase inhibitory activity of Gac extract, vitamin C, and vitamin E.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)