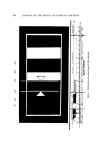
308 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (gas-free) foam overflow, uo is the superficial linear velocity of gas, and v is the kinematic liquid viscosity, t•/p. Group 0t is defined as t•aG/t•82gpA or its equivalent uova/gv?, where •8 is the kinematic surface viscosity, Figure 3 shows the theoretical relationship (9) between oe and • for very dry foam. For wetter foam, the theoretical results are more com- plicated, but can be well approximated by simply multiplying Q ob- tained from Fig. 3 by (1 + 3Q/G) to give a revised Q. For variation in bubble size, r should be replaced by ra.•. For columns of nonuniform cross section, the theoretical development must be modified. 20O 4O ioc 8o 60 2o IC I0 -5 I0 -4 I0 -'• IC• 2 IC[ I I I0 Figure 3. Theoretical relationship (9) between oe and • [or very dry loam The theory also predicts that a very stable foam in a vertical column of uniform cross section under conditions of steady drainage or steady overflow will show a liquid content that does not vary appreciably with level (height). This interesting result has been supported by experi- ment (3, 9, 10). The quantitative predictions of liquid content and overflow rate have been fairly well confirmed too, provided a proper effective t•8 is employed for the surfactant involved (3). In particular, the values of pts ) 104 dyne sec/cm for saponin, bovine serum albumin, and Triton X-100 in water are 4.2, 2.6, and 1.0, respectively (based on the bubble size distributions observed at the column wall, without further correc- tion). Such low effective values for saponin and albumin in comparison to those obtained by surface viscosimetry may reflect, at least in part, the thixotropic or other non-Newtonian behavior of such otherwise rigid surfaces--the average transit time for flow through a PB (from mixing cell to mixing cell) being less than one second.
PHYSICAL ASPECTS OF FOAM 309 FRACTIONATION Overflowing foam involves foam fractionation. Dissolved (or col- loidal) surface-active components of the liquid are adsorbed at the bubble surfaces and carried off by the foam. A surface-inactive com- ponent often can be adsorbed through union with an appropriate sur- face-active collector. Figure 4 illustrates simple foam fractionation, batch and continu- ous, with gas injection. If the submergence of the bubbler in the liquid pool is not too shallow, and the foam is stable, the solute surface excess, r•v, on the bubbles will be approximately in equilibrium with the solute concentration in the pool, C,•. The foam fractionator then operates approximately as a single theoretical (perfect) stage of separation. Overflow Focm Foam Liquid Feed Liquid Overflow Bottoms.. Gas 1 • Gas • (a) (b) Figure 4. Foam fractionation operating in the simple mode: (a) Batch, (b) Flow A solute material balance on the foam gives 3GF•v co = c., + ½2) where CQ is the solute concentration in the overflowing foam after it is collapsed, and 3/r is the ratio of surface to volume for a bubble. By combining eq 12 with a material balance over the entire fractionator under steady continuous operation, one obtains 3GF•v Cw = Cv rF (13)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)