322 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS was undertaken as the result of an observation made in a related program on permanent waving in which the curl retention of hair on mannequins was being measured after the hair had been cold waved with a cross- linking, cationic resin. The dramatic improvements in ease of combing and apparent body of the hair led to the suggestion that the polyamide be applied for this purpose alone, either with or without curlers used for waving. Although discovered while the observer's attention was directed only to curl retention, the effects of the polymer treatment on improved body and manageability are understandable, and might have been predicted. Through autoradiograms of hair treated with radioactively labeled material, this wat4r-soluble polyamide resin was shown to coat the hair fibers continuously. It then cures by reaction with hair or by crosslinking with itself to give greater stiffness and desirable effects on body or apparent fullness. The coated hair fibers should be smoother and, as a result, easier to comb and less subject to snarling. Users re- ported less "flying" during combing, indicating that the resin reduced static charge development. The program undertaken had the objectives of optimizing resin properties, of defining the effects on hair, and of es- tablishing preferred conditions for using the reactivity to assure reten- tion. Such a program must also include a thorough study to assure safety not only to hair but also to skin. In this case, extensive medical studies and in vivo use studies conducted in a related program on cold waving had previously demonstrated safety for this kind of treatment. After some preliminary work on optimum composition, the program ad- vanced rapidly to personal use studies. The resin is a low molecular weight form of an adipic acid/diethylene- triamine polyamide in which epichlorohydrin has been reacted with secondary amines to generate azetidinium functional groups that react readily with sulfhydryl groups in hair protein. For optimum results, the concentration of sulfhydryl groups in the hair is first increased by treat- ment with either sodium bisulfite or ammonium thioglycolate. For most of the work, a thioglycolate salt was used under conditions nor- mally employed for "softening" hair in a cold-waving process. Un- reacted sulfhydryl groups can be restored to their normal disulfide cross- linking form by use of a mild bromate oxidizing agent. Thus, the chemistry involved and procedures employed are very similar to the glycolate "softening" and bromate "neutralizing" steps widely used in cold-waving processes. The important difference is that a resin has been
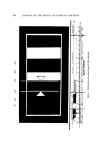
POLYAMIDE RESINS FOR HAIR 323 bonded to the hair to give improved body. The size and number o[ curlers to be used depend on the amount of curl desired. For some purposes, only limited curl is desired and is used for its effect on ap- parent body of the hair, not to give a curl per se. Synthesis of the aminopolyamide and its reaction with epichloro- hydrin to give the reactive azetidinium chloride derivative are shown in Fig. 1. HOOC(CH,04COOH + H•NCH2CH2NHCH2CH•NH2 H [NH•(CH2) 4 •N m cg2cm2$-- cg2cg 2 Ix o o Epichlorohydrin © c1 © [NHC(CH,)4CNHCH2CH•/N. CH2CH2]x O O aC•c•CH2 I OH •igure 1. Aminopolyamide synthcsis and reaction with epichlorohydrin As shown in Fig. 2, ammonium thioglycolate reacts with the disulfide groups o[ hair to generate sulfhydryl [unctionality. Reaction o[ the resin with sul[hydryl groups is shown in Fig. 3. The sketch shows only one o[ the thioether linkages. Since both resin and hair protein are poly[unctional, the resin may be bonded at more than one point. Conditions for applying the resin to hair and completing the reac- tions were studied by use o[ 2-g hair tresses, which were also useJul [or observing the effects on ease o[ styling, style retention, improved body, S S S S S S S S SHCH2C02NH4 H I H s s s Disulfide crosslinked Protein with disulfide hair protein linkages partially reduced to sulfhy dryls Fi,•ure 2. Reaction of ammonium thio•lycolate to generate reactive sulfhydryl groups
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)