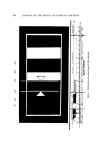
ANALYSIS OF SUNSCREENS 335 A close look at the details of the energy versus wavelength curve (Fig. 1) shows the very rapid decline of the energy level with increasing wave- length (1). This is not a uniform, steady decline of energy level as the wavelength increases. There is a gTeat irregularity of the energy level (2, 3) in the portion of the solar uv curve which interests us (Fig. 2). Note that the erythemal and tanning ranges overlap. This will be of interest later, since some of the "border" values obtained will contribute both to the "erythemal values" and to the total "tanning radiation transmitted." The highest energy level is between 295-305 mt•, which area is responsible for most of the injury caused by sunburn. For this reason, chemists have been busy for about 40 years, searching for a sunscreen whose absorption curve would match most closely this irregularity of the energy level curve of the ultraviolet spectrum. Hundreds of screening compounds have been suggested, ranging from natural oils and greases to synthetic organic chemicals. The four most widely used screens today, all of them synthetic products, are (Fig. 3): 1. amyl-p-dimethylaminobenzoate • 2. 2-ethoxyethyl-p-methoxycinnamate* 3. glyceryl-p-aminobenzoate* 4. homomenthyl salicylate• In order to correlate the changing intensity of solar radiation with equivalent erythemal response of human skin, a unit of "erythemal flux" has been devised. This unit is called "E-viton" (10 t•W/cm 2 of wave- length 296.7 mt• radiation) (4). EXPERIMENTAL METHODS AND RESULTS Preliminary Testing o.f Suntan Products Spectrophotometric procedures are used for identification and are usually conducted by comparing the general shape and exact position(s) of the product's peak(s) with a spectrogram of a known uv absorber. This is sometimes followed by a programmed gas chromatography (gc) run, to establish whether a single compound or a combination of screens is in- volved. (SE 3011 has been found a very reliable column, with the tempera- * Escalol 506, Van Dyk & Co., Inc., Belleville, N.J. 07109. t Giv-Tan F, Givaudan Corp., Clifton, N.J. 07014. $ Escalol 106, Van Dyk & Co., Inc. õ Orbis Product Corp., 475 10th Ave., N.Y. 10018. [[ 6-ft column, 10% silicone gum rubber, 60-80 mesh, from Hewlett Packard, Avondale, Pa.
336 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Figure 2. Erythemal flux of midsummer sun ture programmed from 200/300øC at 10øC/min.) Since sunscreens are mostly aromatic esters, they need not be silanized beforehand. One mi- croliter injected at 10 a X 16 attenuation usually gives a good resolution. Quantitative Evaluation of a Sunscreen Figure 4 shows the spectrophotometric curve of one commercial prep- aration. This curve was made on a Beckman DB double-beam spectro- photometer, having 2 matched quartz cells. Samples should be well mixed before weighing. In the case of aerosol spray sunscreens, precool- ing of both the can and the receiving container is advisable to minimize evaporation loss during transfer. All glassware used must be cleaned in advance with hot (80-100øC) chromic acid mixture and rinsed several times with hot water, then finally with alcohol. Quartz cells must be cleaned also in hot chromic acid mix- ture followed by rinsing with distilled water, then alcohol. The utmost importance of clean glassware. and especially of the quartz cells, must be
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)