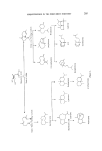
208 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Oisobolene Figure 5. Zingiberene has a warm, woody-spicy tenacious odour with deep sweetness.' Its structure as shown was assigned by Eischemosher and Schinz and also by Mills (20, 21). Several syntheses of (+) zingiberene have been reported but the most interesting approach is based on citronellal and can lead to' the optically active hydrocarbon (22, 23). ar-Curcumene has been synthesized by Honwad and Rao (24, 25) but has an uninteresting odour. Zingiberene ar-Curcurnene Figure 6. a-Bisabolo! occurs in oil of camomile (26, 27) and the racemic form is prepared by acid-catalysed cyclization of nerolidol (16). It can be used as a fixative and blender in many formulations with interesting results. Another interesting member of the group is a primary allylic alcohol lanceol which occurs in the oil of Santahim lanceolatum (21, 28). •- Bisobolol Lo nceol Figure 7. Germacranes, a group of 10-membered ring compounds, were postu- lated by Ruzicka, Eschenmoser and Heusser (29) as intermediates of crucial significance in the biogenesis of the elemane-, eudesmane-, and guaiane-type sesquiterpenoids from farnesyl pyrophosphate (29). However, no member of this group was actually isolated and characterized until germacrone and
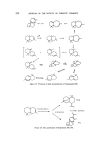
SESQUITERPENES IN THE PERFUMERY INDUSTRY 209 pyrethrosine were characterized by Ognyanov et al and Barton and de Mayo respectively in 1957 (30, 31). Since then several other compounds belonging to this group have been isolated and characterized by careful work-up. Sorm has recently published an excellent review of the chemistry of germacranes (32). OAc Ger toocrone Pyrethrosine Figure 8. Germacrone was isolated from oil of Geranium macrorhizum in 50•o yield (30). It has a faint, sweet-woody, somewhat herbaceous odour of extraordinary tenacity. Although the ketone is not offered as a pure chemi- cal, the oils rich in this material have been suggested to be useful as a modifying fixative in ambre, chypre, and mossy fragrance types. The pure ketone itself blends well with the ionones, geranium, ambergris, vetiver, and cedarwood types (13). The hydrocarbons germacrene A, B, C and D offer interesting synthetic possibilities of obtaining various well-known sesquiterpenes which are crucially important in simulated essential oils. Ge r macrene -A Germacrene -B Germacrene-- C Germacrene-- D Figure 9. A variety of acid-catalysed and photochemical transformations to systems such as eudesmanes, copaene, ylangane, bourbonane (present in the essential oil of geranium bourbon), cadinane, and muurolane are known and illustrate the biogenetic significance of this group (33). The elemane group consists of a number of hydrocarbons and oxygenated derivatives including lactones and occur widely in many essential oils. These compounds are closely related to eudesmanes and germacranes, and it is generally supposed that many members are probably artefacts of
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)