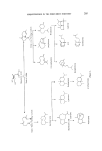
216 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Longifolene (I) HCHO AcOH (2)NaOH Figure 19. ••CH20 H' t•-- Hydroxymethy I Io ng ifolen e of recent interest in the production of new monoterpene perfumery chemi- cals (67-69), e.g. dihydromyrcene cyclic esters. Application of these reaction conditions to longifolene produces longibornan-9-ol formed by rearrange- ment and transannular hydride shift (70). A recent patent claims this formate to be of value in perfumery (71). •\\ o II (I) HCOH (2) NeOH Long i fol ene Longiborna n-9-ol Longifolene Isolongifolene Figure 20. Early attempts at longifolene acetoxylation did not give good yields of acetates but an isomer of longifolene was produced called isolongifolene (72). The structure of isolongifolene was established by Ranganathan et al. (73) who later completed a total synthesis of the hydrocarbon (74). [solongifolene has proved to be of more commercial interest than longifolene as simple methods of oxidation yield non-acidic products. If longifolene or isolongifolene are treated with zinc chloride at high temperatures then 1,1-dimethyl-7-isopropyltetralin is formed (75). Acetyla- tion of this tetralin gives the methyl ketones these are said to have a fine although weak musk odour. The aldehydes have a stronger musk odour but the propionyl compound is odourless (75). Epoxidation of isolongifolene with peracids yields the •z-epoxide (76). This epoxide is of little perfumery interest but rearrangement of the epoxide produces the saturated ketone 8-oxo-7-13-H-isolongifolane which has a desirable woody odour and has found use in perfumery. Epimerization of
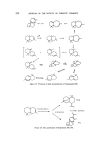
SESQUITERPENES IN THE PERFUMERY INDUSTRY 217 Longifolene A% 0 •r /•• ZnCIz //,• [solongifo!ene Figure 21. this ketone to the 7(a)H derivative is simple and this ketone is also of perfumery use as it possesses a sweet-woody odour. The rearrangement of isolongifolene epoxide has been reported (77-79) to yield two [3, T-unsaturated alcohols. No patents have been published on the rearrangement of the epoxide under most reaction conditions these alcohols are formed in less than 50•o yield, the main product is 8-oxo-7-[3- H-isolongifolane (Fig. 22). iso Longifolene-a -epoxide Figure 22. o 8- o xo -7 -/•-H-isolongifolane The sterochemistry of isolongifolene is a subject of academic discussion (76-79) and it is still not known beyond all doubt if the epoxide is, in fact, a- or [3. In the author's opinion, the a-structure is the more probable (80). Sodium dichromate oxidation of isolongifolene yields a complex mixture of ketones with a woody, vetiver type of odour. This mixture has found use in perfumery (81). Allylic oxidation of isolongifolene with tertiary butyl peracetate gives a mixture of 9a- and [3-acetoxy-isolongifolene. This has a woody, vertiveryl acetate odour and has found use in perfumery (82) (Fig. 23). Isolongi folene 9 Acetoxy - i solongi folene Figure 23.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)