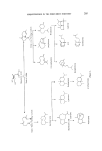
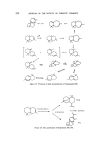
224 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Figure 33. Products of acid isomerization of thujopsene (95). 52% Acetylof ion + 5other i$omers Fieure 34. The acetylation of thujopsene (96, 97). 9%
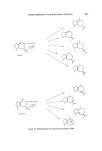
SESQUITERPENES IN THE PERFUMERY INDUSTRY 225 o - Patchoulene /• -- Patchoulene 7'- Patchoulene o- Guaiene Caryophyllene B -- Guaiene Patchouli alcohol , Figure 35. Some constituents of patchouli oil. the synthesis involves a large number of steps. [3-Patchoulene is first syn- thesized and then converted to the a-patchoulene structure and hence patchouli alcohol. No commercial synthesis of this alcohol appears possible at the present. Patchouli oil is interesting as it is one of the few oils obtained by steam distillation and is found to contain sesquiterpene alkaloids (102). These alkaloids are formed from the sesquiterpene structures of two hydrocarbons found in patchouli oil, [I-patchoulene and a-gauiene thus they have been called patchoulipyridine and epiguaipyridine. The structures of these products, present in the oil to the extent of 0'1•o were determined by physical organic techniques (102) and confirmed by the synthesis of pat- choulipyridine and dihydro-epiguaipyridine. Patchoulipyridine was syn- thesized from [•-patchoulene by the action of hydrazoic acid and dehydro- genation of the resulting product. Dihydro-a-guaiene was synthesized (102) from guaiol and subjected to the same reaction steps to yield dihydro- epiguaipyridine. No odour descriptions have been given for these two alkaloids. An alternative synthesis (103) of patchoulipyridine has been developed by
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)