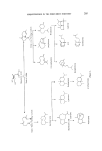
220 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Canadian workers have devised a route for the synthesis of camphere- none. This sesquiterpene is a valuable intermediate for the synthesis of a number of sesquiterpenes and in two steps it may be converted either to a-santalene or [I-santalene (87, 88) (Fig. 28). /•- Santalene i a-$antalene Figure 28. This synthesis might have been of commercial value but at the key cyclization stage two compounds are produced, one of which gives santalene and the other epi-santalene. An alternative approach was examined by American workers who synthesized 3-methyl-norcamphor from the Dieis-Alder product of methyl cyclopentadiene and ethylene. The construction and addition of the side chain to the camphene structure was more complex and again this synthesis does not offer, at present, any commercial possibility (89). A more direct synthesis has been described. The key step was the Dieis- Alder addition of geraniol to cyclopentadiene low yields at this stage appear to have discouraged exploitation of this path (90). The lactonization of camphene-8-carboxylic acid has been reported to yield a lactone with the correct arrangement of functional groups to be a santalene intermediate. Here again the key step produces not only the desired santalene structure but also the epi-santalene structure (91).
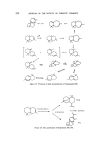
SESQUITERPENES IN THE PERFUMERY INDUSTRY 221 As a result of these synthetic studies the odours of a number of santalol derivatives have been reported (89). Dihydro-l%santalol is said to have a sandalwood odour but the e-isomer is said to be much weaker. Tetrahydro- [%santalol has no sandalwood character and has an uninteresting weak woody odour. There is no doubt that the industry would find a synthetic santalol a most welcome addition to the perfumers shelf. It is not possible to say how long it will be before it arrives there. Cedarwood oil is one of the essential oils whose production exceeds 100 tons/year. The oil is not only of direct use in perfumery a number of derivatives from the oil have also found a wide use. Although some of these derivatives have been given names in the technical press which would suggest that they are definite chemical entities, many of them are mixtures (e.g. cedrenol) isolated from the oil or the products of reactions on such mixtures (e.g. acetyl cedrene). Thujopsene and cedrene are the two main sesquiterpene hydrocarbons found in the oil [•-cedrene, [•-chamigrene, widdrene, isowiddrene, a- chamigrene, cuprenes and cuparene have been found in smaller quantities (92) (Fig. 29). Cedrol is the main alcohol constituent of the oil psuedo cedrol, primary cedrol and widderol are also found in the cedarwood oil (92) (Fig. 30). a- Cedrene B-• Cedrene •-Ckamigrene Wh:tdrene isoWiddrene a - Chamigrene Cuprene ]Z Cuprene Cupa rene Thuj o pse ne Figure 29. Some hydrocarbons from cedarwood oil.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)