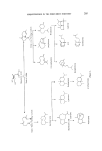
214 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 4 - Epinootkatone Fruity, woody, bitter B-y- Nootkatone Grapefruity- bitter Nardostachone F'ruity- bitter I, I 0 -Dihydronootkatone Fruity-bitter iso- Noot katone Woody 4-epi -isonootkatone Woody I 1,12- Dihydronootkatone floral-woody Figure 16. H Tet rahyd ron ootk atone floral-woody a --Vetivone 0 • • /• -Vetivone CH20H CH20H Tr[cyclovetivenol (KhusJmol) Figure 17. Bicyclovet ivenol
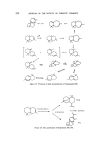
SESQUITERPENES IN THE PERFUMERY INDUSTRY 215 quality, vetiver oils contain 45-65•o free sesquiterpene alcohols bicyclo- and tricyclovetivenols. The commercial product is a mixture of these two vetivenols and has a warm, sweet, mildly earthy-balsamic and extremely tenacious odour. These alcohols blend well with ionones, styrax, sandalwood and various materials for oriental and woody bases or perfumes (13). The corresponding aldehydes are known to have an olibanum type odour. The mixed acetates obtained by the acetylafion of the above alcohols or by isolation from essential oil are sold under several trade names. The sweet- dry, fresh-woody odour with excellent tenacity allows its use in all types of perfumes (13 ). Longifolene, a hydrocarbon belonging to the longifolane group, is one of the few sesquiterpenes commercially available in quantity. Much interest has been shown by the industry in the new materials that chemical in- vestigation into longifolene chemistry has presented to the perfumer. Longifolene was first isolated by Simonsen (59) from Pinus longifolia and the search for commercial uses for the hydrocarbon extends back 50 years. The structure was established by Moffett (60) and Ourisson (61) and commercial exploitation has followed this breakthrough. The structure of longifolene has been confirmed by a total synthesis (62) but such syntheses are of academic interest only. Two simple longifolene derivatives are on the perfumer's shelf at the moment: acetyl longifolene and hydroxymethyl longifolene. Acetyl longi- folene (63) is prepared by the Friedel-Crafts reaction and has a woody- musky ambergris odour reminiscent of acetyl cedrene. Longifolene Figure 18. Acetyl Iongifolene The Prins reaction has been of use in the production of perfumery chemicals from monoterpenes (Nopol, Patchenol) and application of this reaction to longifolene produces to-hydroxymethyl longifolene (64, 65). This alcohol is of use in perfumery. The simple methods of longifolene oxidation yield complex mixtures with sesquiterpene acids predominating (66). Direct oxidation of longifolene has not been of great commercial interest. Formylation reactions have been
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)