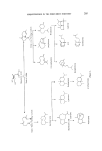
218 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS The rapid commercial exploitation of longifolene in the last few years illustrates the interest shown by perfumers in new sesquiterpene products. With the considerable academic and commercial interest being shown in this hydrocarbon, further developments of interest to perfumers may well be expected. Sandalwood oil is much used in perfumery and has a large production its odour is too well known to be described in this paper. The main con- stituents of the oil are known (83) tz-santalene had a sweet-woody odour of excellent tenacity J3-santalene has a similar odour but is said to be less sweet than the tMsomer. tz-Santalol is considered to have the refined sweet- woody, tenacious sandalwood odour. Impure tz-santalol isolated by distil- lation from sandalwood oil is the santalol of commerce. J3-Santalol is said to have a similar odour but it occurs to a smaller extent in sandalwood oil and samples may have been contaminated with the tz-isomer. Perfumery opinion is that the tz-isomer is the preferred isomer (Fig. 24). (•-- Santa lene •- Santalene a- $antalol B--$antalol CH•OH Figure 24. The santalyl structure is related to the structure of 1ongifolene if it were possible to open the seven-membered ring of longifolene one would have a santalene structure. The sterochemistry is such that the opposite optical isomer to the natural santalene would be produced (Fig. 25).
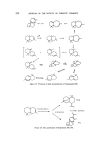
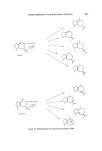
SESQUITERPENES IN THE PERFUMERY INDUSTRY 219 Figure 25. Sandalwood is a moderately expensive essential oil and its typical bouquet continues to be favoured by the perfumery industry. The search for sandalwood odours have therefore been intense. A number of chemicals with a sandalwood odour have been made. The so-called terpeno-phenols manufactured by the condensation of phenols with camphene followed by hydrogenation (84) have found application in a number of sandalwood bases. These have found a use in the industry but have not depressed the demand for the natural oil. The last addition to the sandalwood odours is Osyrol (and its homologs), and although this monoterpene is not a direct replacement for a-santalol, this speciality has a fine sandalwood odour and with its good performance in the middle notes it will be of wide interest in the formulation of perfume compounds where the sandalwood character is desired (85) (Fig. 26). Figure 26. The commercial synthesis of sesquiterpenes has yet to be achieved but much progress has been made in the academic synthesis of these compounds. Starting from ct-bromocamphor French workers were able to synthesize a- and 13-santalol in nine steps (86). The reaction sequence is too long for this synthesis to be of commercial interest. Figure 27.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)