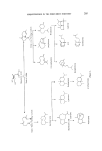
SESQUITERPENES IN THE PERFUMERY INDUSTRY 213 Several stereospecific syntheses of (+)-nootkatone have been reported in the literature. Pinder and Odom (49) annelated m-dihydrocarvone with trans-3- pent-en-2-one to obtain nootkatone, which was also synthesized by Marshal and Ruden by a multistep synthesis (50). A recent patent describes the following synthesis (51). o o thyl Formate Acetone MeMgI • ,. Cu I Figure 15. •- Noot katone Nootkatone has also been the subject of detailed sensory properties. Ohloff and Giersch (52) studied the relationship between odour and structure by comparing the odour and taste of various nootkatone isomers and their derivatives. It was found that those compounds with a fruity odour have a bitter taste, whereas those which are devoid of fruity smell have no taste. Furthermore, the double bond remotely situated from the carbonyl function is of special importance in regard to the odour and taste. Stevens, Guadagui and Stern (53) and Teranishi (54) also arrived at similar conclusions, but add that nootkatone isomers have different odour qualities but have only small differences in potencies. The compounds belonging to vetispirane and tricyclovetivane are derived mainly from the essential oil of Vetiveria zizanoides, a perennial grass which grows wild in India, Ceylon, Burma, Reunion Island, and several other countries. The oil, which has been used in perfumery since antiquity, consists mianly of sesquiterpenes. These sesquiterpenes are a- vetivone (55), •-vetivone (56), tricyclovetivenol or khusimol (57), bicyclo- vetivenol (58), and vetivenyl acetates. Originally these compounds were assigned wrong structures which have been refuted only recently and the revised structures are as shown below. t•- and •-Vetivones are used in per- fumery as a mixture derived from the ketone fraction of vetiver oil. They possess an odour which is very much reminiscent of the parent oil but more tenacious. These ketones can be a valuable part of oriental type formula- tions (13). The chemistry of t•-vetivone is closely related to that of noot- katone and hence is also known as isonootkatone. Due to this relationship with nootkatone it would be surprising if we do not see a synthetic material commercially available in the near future. Depending upon their origin and
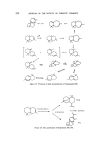
214 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 4 - Epinootkatone Fruity, woody, bitter B-y- Nootkatone Grapefruity- bitter Nardostachone F'ruity- bitter I, I 0 -Dihydronootkatone Fruity-bitter iso- Noot katone Woody 4-epi -isonootkatone Woody I 1,12- Dihydronootkatone floral-woody Figure 16. H Tet rahyd ron ootk atone floral-woody a --Vetivone 0 • • /• -Vetivone CH20H CH20H Tr[cyclovetivenol (KhusJmol) Figure 17. Bicyclovet ivenol
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)