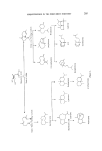
SESQUITERPENES IN THE PERFUMERY 1NDUSTRY 227 ,01 •04 AcOH Guaiol O, 01, M H2•S 04 AcOH Bul nesol Figure 37. Hydrocarbons l•rom guaiol and bulnesol. (104).
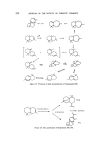
228 JOURNAL OF TIlE SOCIETY OF COSMETIC CHEMISTS to be of value in perfumery (106). It has a distinctive odour of the warm vetiver type with a warm dry spicy note which is reminiscent of black pepper oil. The cyclic ether has also been reported in guaiacwood oil. A cyclic ether has been isolated from sandalwood oil (107), and it is possible that sesquiter- pene ethers will be found to be of some considerable value in perfumery. Guaioxide Sandalwood ether Figure 38 In conclusion it can be said that sesquiterpenes offer a great challenge to the Organic Chemist because of their structural complexity. They are of crucial importance to the perfumery industry as they occur widely in essential oils and individual chemicals offer a wide spectrum of interesting odour types. Therefore, any advancement in the difficult problems of skeletal construction and stereochemical control necessary for such syntheses would have a significant impact on the future of the industry. Undoubtedly during the recent years many new and elegant methods of synthesis have been introduced but unfortunately the majority are of academic interest only. However, one hopes that, in addition to this progress, a better understanding of enzymatic reactions coupled with their commercial exploitation may provide an answer to some of the problems. (Received: 13th February 1973) REFERENCES (1) Parker, W., Roberts, J. S. and Ramage, R. Quart. Rev. 21 331 (1967). and references therein. (2) Popjak, G. and Cornforth, J. W. J. Blochem. 101 553 (1966). (3) Sorm, F., Leziva, J. M., Arnold, Z. and Pliva, J. Collect. Czech. Chem. Commun. 14 699 (1950). (4) Sorm, F. and Arient, J. Collect. Czech. Chetn. Cornrnun. 15 175 (1950). (5) Sorm, F., Zaoral, M. and Herout, V. Collect. Czech. Chern. Cormnun. 16 626 (1951). (6) Peyron, L., Benezet, L. and Gamere, J. Bull. Soc. Chirn. Ft. (8), 3038 (1967). (7) Naves, Y. Helv. Ch#n. Acta, 49 1029 (1966). (8) Brieger, G. J. Org. Chetn. 32 3720 (1967). (9) Hesse, A. and Zeitschel, O. J. Prakt. Chern. (ii) 66 504 (1902). (10) Katsuragi, H. Koryo No. 22 23 (1952). (11) Calveano, Maria. Essenz Deriv. Agrutn. 36 (4) 237 (1966).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)