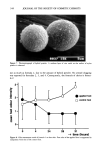
J. Soc. Cosmet. Chem., 40, 309-320 (November/December 1989) Selective removal of sebum components from hair by surfactants JANE CLARKE, CLARENCE R. ROBBINS, and BRENDA SCHROFF, Colgate-Palmolive Research Center, 909 River Road, Piscataway, NJ 08854. Received August 15, 1989. Synopsis The detergency of three surfactants, sodium laureth 2-sulfate (SLES-2), ammonium lauryl sulfate (ALS), and sodium octeth-1/deceth-! sulfate (SODS-I), was measured variables examined were soil/wash cycles plus sebum component vs total sebum removal. After one soil/wash cycle SLES-2 cleans all sebum compo- nents from hair equally well (90%). ALS is not as good, and SODS-! is poor for all fractions. With extended use (ten-cycle data), SLES-2 remains superior for all components (90% removed), but the behavior of ALS and SODS-1 are substantially different from their one-cycle behaviors. Analysis of tresses washed with ALS under test and simulated use conditions suggests a build-up of fatty acid components on hair this is interpreted in terms of a hard water ion/fatty acid interaction. Extended use data of SODS-1 show increased removal for all components when compared to the one-cycle data, suggesting either a soil release mechanism or inhibition of soiling. We hypothesize that a technique that provides a rapid assessment of total sebum removed from hair by a detergent can be used to screen surfactants. However, to model extended use behavior, it is useful to monitor the removal of sebum components. INTRODUCTION Effective formulation of hair cleaning products begins with an understanding of the substrate. Perhaps of equal or even greater importance is the type of soil found on the substrate and how it is bound to the fibers. Human hair has a chemical composition, physical properties, and histological structure similar to other keratin fibers. However, the cleaning of hair presents a different, and possibly more difficult, problem because of safety restrictions. The use of fairly low temperatures and short cleaning times adds further restrictions. In comparison, products for cleaning textiles do not have to meet such restrictive criteria. Soils on human hair can be divided roughly into four groups: (a) Hair lipid, a fatty material composed mainly of sebum (from sebaceous glands) and lipids (from skin surface cells). (b) Proteinaceous matter from cell debris and sweat. (c) Extraneous materials from a polluted environment (soot, hydrocarbons). 3O9
310 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (d) Hair product soils, e.g., conditioners, hair sprays, mousses, gels, etc. The perception of dirty or oily hair is probably attributable to hair lipids. These mate- rials may be sticky and can act like a "cement," causing various particulates to stick to the hair surface. Sebum production is variable (1). This variation is documented to be seasonal, daily, and due to hormonal activity, with changes from preadolescence, through puberty, and into old age. Compositional changes also occur with both subject age (1), and as the sebum ages (1), after distribution on the hair. Furthermore, evidence exists for the classification of hair sebum into two types: external or surface sebum, and internal sebum (2). External sebum contributions combine with the physical properties of the hair fibers (curliness, diameter) to furnish hair with an oily appearance. It is reasonable to assume that the external sebum, which is easily extractable into lipid solvents, can be shampooed off, while the internal lipid is more difficult to remove. In fact, very strong extraction procedures and enzymatic hydrolysis of hair keratin (2) is needed to remove this material. The exact origin of the internal lipid is under debate, but Koch et al. (2) have provided evidence that most of the components are those found in external lipid. This suggests at least partial origination of these lipids from the sebaceous glands. Several published papers detail methods for extracting hair lipid (external and internal) both in vitro and in vivo (1-4) and for quantifying the data. There are obvious disadvan- tages in collecting lipid in vivo by solvent extraction. Gravimetric analysis, because of the small quantities involved even after in vitro extraction, requires sensitive weighing equipment and care. Several authors have carried out compositional analysis of extracted lipM/sebum. Shaw (5) used gravimetric and spectrophotometric methods to assess total lipid, a fluorimet- ric technique to determine cholesterol, and thin layer chromatography (tlc) and gas- liquid chromatography (glc) to distinguish between major components of the lipid. Koch (2) determined the total amount and composition of extracted sebum by high pressure liquid chromatography (HPLC). Breuer (6) also reports data for quantifying components of extracted sebum using an HPLC technique. Thompson et al. (7) have described a gas chromatography system for the analysis of sebum components extracted into hexane from hair (in vitro). This work arose from our use of a modification of the Thompson et al. technique to determine the extent to which a test measuring total sebum removal from wool (by surfactants) was applicable to predicting surfactant performance against sebum, and to investigate certain surfactants of proprietary interest. Thus this work builds upon the published study of Thompson et al. We believe that the knowledge acquired in deter- mining surfactant selectivity for removal (cleaning) of sebum components from hair can provide important guidance for formulating shampoos and other hair cleaning products. MATERIALS AND METHODS ARTIFICIAL SEBUM The artificial sebum used in all experiments was prepared according to the Spangler formula (8) shown in Table I.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)