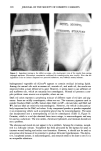
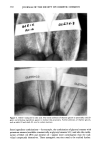
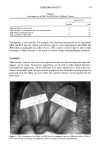
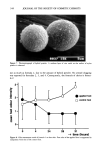
346 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS logical fluids (5). Along with low-molecular-weight compounds containing nitrogen and sulfur, short-chain fatty acids seem to comprise a considerable portion of human body malodors. The best method to efficiently eliminate short-chain fatty acids was considered to be through chemical reaction converting them into their corresponding odorless metallic salts. Out of the several chosen candidates, zinc oxide was found to be most suitable. Taking into account the several shortcomings that zinc oxide possesses, we have devel- oped a hybrid powder consisting of a spherical nylon resin as the core whose surface is uniformly covered with fine particles of zinc oxide. This hybrid powder overcomes zinc oxide's drawbacks, especially those encountered upon formulating it into deodorant products, without sacrificing any of its deodorizing power. The body odor quenchers formulated with hybrid powder were assessed on subjects with strong foot and axillary odor, and were found to be more efficacious in eliminating malodors as compared with conventional antiperspirants and deodorants. The hybrid powder body odor quencher is a novel deodorizer that theoretically not only prevents the generation of body malodor as conventional products do, but also chemi- cally "quenches" body malodor once formed from short-chain fatty acids. This concept is applicable to body odors from regions other than the foot and axilla, provided that the key odor components are short-chain fatty acids. REFERENCES (1) F. Kanda, E. Yagi, M. Fukuda, K. Nakajima, T. Ohta, O. Nakata, and Y. Fujiyama, Elucidating body malodour to develop a novel body odour quencher, 15th IFSCC International Congress Preprints, Vol. 3, 529-562 (1988). (2) H. Boelens, H. G. Haring, and D. de Rijke, Threshold values of and human preferences for 4-ethyl octanoic and 3-methyl butanoic acid, Perfum. Flav., 8, 71-74 (1983). (3) G. Preti and G. R. Huggins: Cyclical changes in volatile acidic metabolites of human vaginal secre- tions and their relation to ovulation, J. Chem. Ecol., 1, 361 (1975). (4) N. Goetz, G. Kaba, D. Good, G. Hussler, and P. Bore, Detection and identification of volatile compounds evolved from hair and scalp using headspace gas chromatography, J. Soc. Cosmet. Chem., 39, 1-13 (1988). (5) T. L. Perry, S. Hansen, S. Diamond, B. Bullis, C. Mok, and S. B. Melacon, Volatile fatty acids in normal human physiological fluids, Clin. Chim. Acta, 29, 369-374 (1970).
j. Soc. Cosmet. Chem., 40, 347-365 (November/December 1989) Synergism of preservative system components: Use of the survival curve slope method to demonstrate anti-Pseudomonas synergy of methyl paraben and acrylic acid homopolymer/copolymers in vitro D. S. ORTH,* C. M. LUTES ANDERSON, D. K. SMITH, and S. R. MILSTEIN, The Andrew Jergens Company, 2535 Spring Grove Avenue, Cincinnati, OH 45214. Received July 2, 1989. Synopsis The survival curve slope method allows determination of synergy in multicomponent systems when the slope (i.e., rate of death of the population of test organisms) is a larger negative number than the sum of the slopes of each of the components. This method was used to demonstrate anti-Pseudomonas synergy of methyl paraben (MP) and acrylic acid homopolymer/copolymers in vitro. Preservative efficacy testing of nonionic lotions containing 0.2% MP and 0.2% acrylic acid homopolymer/ copolymers revealed anti-Pseudomonas synergy against P. aeruginosa, P. putida, P. fluorescens, and P. stutzeri. Addition of 0.1% CaC12 to the lotion caused significant increases in D-values and eliminated the anti-Pseu- domonas synergy. Similar patterns of synergy were observed in lotions containing 0.2% MP q- 0.2% carbomer 934, 941 or acrylates/C10-30 alkyl acrylate cross polymer (1342) and in tap water containing 0.2% MP q- 0.01% Na2EDTA. The anti-Pseudomonas synergy observed with MP and neutralized acrylic acid homopolymer/co- polymers is probably related to chelation of divalent metal ions and similar to permeabilization synergy reported for preservative action by EDTA. INTRODUCTION Preservative efficacy testing is performed to determine the type and minimum effective concentrations of preservatives required for products to meet acceptance criteria (1). Testing is needed for each product because the physicochemical composition of a for- mula may enhance or reduce the antimicrobial effectiveness of preservatives. When designing the preservative system of a product (2,3), it is desirable to select compounds that enhance the antibacterial action of the preservative system. Synergism is observed when the effect produced by the combination of components is greater than the sum of the effects of each component taken separately. Synergy of antimicrobial preservatives has been reported by several workers (4-7). *Current Address.' Neutrogena Corporation, 5755 West 96th Street, Los Angeles, CA 90045. 347
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)