336 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS reported that short-chain fatty acids contribute considerably to both foot and axillary odor (1). Especially in the case of foot odor, isovaleric acid was found to be the key odor component responsible for the malodor. As for axillary odor, a particular key odor component remains yet to be identified, although short-chain fatty acids of compara- tively long carbon chain (C6) seem to comprise a considerable portion of the malodor. It is well known that the method of choice in eliminating short-chain fatty acid mal- odors is to convert volatile short-chain fatty acids into their corresponding odorless nonvolatile fatty acid metallic salts. In this study, ingredients capable of converting short-chain fatty acids into their me- tallic salts were investigated by headspace gas chromatography (HS-GC). Furthermore, deodorant products formulated with such ingredients, which hopefully will not only prevent but also act directly upon malodor already formed, were compared with con- ventional products for their ability to efficiently quench foot and axillary odor. EXPERIMENTAL HEADSPACE GC ANALYSIS FOR EVALUATING QUENCHING ACTIVES Equilibrium headspace gas chromatography was employed to assess the ability of various compounds to efficiently quench short-chain fatty acids. HS-GC is unique in that only the vaporized portion of the sample is introduced into the GC. The method permits analysis of volatile chemicals without having to introduce the total sample matrix into the GC. The sample matrix may well contain nonvolatile compounds that are neither amenable nor desirable for GC operation. Isovaleric acid was chosen to represent the short-chain fatty acids since it was found to be the key odor component of foot odor and also because of its extremely low olfactory threshold level (2). Quantita- tive comparison among the candidates should easily be made since the concentration of isovaleric acid in the vapor phase should be directly proportional to the GC peak area obtained. Approximately 80 mg of the candidate was accurately weighed in a glass vial especially designed for the headspace gas chromatograph, to which one ml of 0.5% isovaleric acid aqueous solution was added. The vial was tightly closed and placed inside an ultrasonic generator for five minutes for sample dispersion. It was then placed inside an oven maintained at 60øC for 60 minutes to allow isovaleric acid vapor to equilibrate in the headspace of the vial prior to analysis. The vial was introduced into a Perkin Elmer SIGMA 3B headspace gas chromatograph equipped with a flame ionization detector and a three-foot glass column packed with 10% FFAP. The HS-GC was operated at a column temperature of 150øC isothermally. The headspace of the vial was automatically pressurized for four minutes, after which it was forced into the carrier gas flow. The GC peaks were recorded and the peak area was calculated in arbitrary units using a Hewlett Packard HP 3380A integrator. For each candidate, three consecutive GC runs were acquired, and the mean peak area was em- ployed for the calculation explained later on. To check the stability of the GC, the standard isovaleric acid aqueous solution was measured once in every five sample runs. Each candidate was evaluated by calculating a value expressed as "isovaleric acid con- sumption/mg ingredient." An example of how to calculate the isovaleric acid consump-
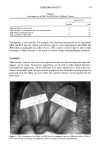
NOVEL POWDER DEODORANT 337 peak area 233612 (arbitrary unit) -i-Ingredient A (80mg) ak area 96281 HSGC of standard iso-valeric acid solution Iso-valeric Acid Consumption_233612--96281 Value of Ingredient A 80 =1717(counts/mõ ingredient) Figure 1. Calculation of isovaleric acid consumption values. Upon addition of a quenching ingredient, the GC peak area of the standard isovaleric acid solution decreases. tion value is shown in Figure 1. The larger the value, the greater the efficacy of the ingredient to quench isovaleric acid odor. CONFIRMATION OF THE QUENCHING MECHANISM BY FT-IR Fatty acids in the free form and metallic salt form are readily distinguishable by Fourier transform infrared spectrophotometry (FT-IR), since they exhibit characteristic absorp- tion bands at different wave numbers. Therefore, the speculated quenching mechanism in which volatile short-chain fatty acids are converted into metallic salts was confirmed by FT-IR. To a mixed aqueous solution (0.1%) of propionic, isovaleric, and caproic acids, resembling that of a sweaty body malodor, zinc oxide was gradually added until excess zinc oxide started to precipitate. The excess zinc oxide was filtered, and the tiltrate was evaporated to dryness in vacuum. An FT-IR spectrum of the resulting res- idue in the form of a KBr tablet was acquired using a Biorad Qualimatic FT-IR, scan- ning a range of 4000 to 400 cm-• FORMATION OF A ZINC OXIDE/NYLON HYBRID POWDER Although zinc oxide is a widely used cosmetic ingredient, it possesses a couple of unfavorable shortcomings that derive from its aggregating property. Even though some commercially available zinc oxides are claimed to be as small as 0.1 •m in particle size, they readily cohere to form massive lumps, as shown in Figure 2. This is said to be due to the electrostatic behavior of zinc oxide, and can thus easily lead to clogging of aerosol
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)