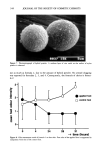
340 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS in the figure, foot odor was evaluated five times during an assessment. During the two days, the subjects were allowed to bathe but not permitted to use soaps or deodorants of any kind. The same assessment was carried out on all four formulae. EFFICACY OF QUENCHERS FORMULATED WITH HYBRID POWDER ON AXILLARY ODOR Efficacy of quenchers formulated with hybrid powder was assessed on axillary odor as well. A panel of 20 patients (three men, 17 women, average age 30), suffering from strong axillary odor, was selected from hospitals and universities in Japan. Double- blind trials were made on body odor quencher A (a conventional formula containing aluminum chlorhydrate as active ingredient + hybrid powder, equivalent to formula 2) and body odor quencher B (a conventional formula containing only aluminum chlor- hydrate as active ingredient, equivalent to formula 4). Trained olfactory assessors eval- uated the efficacy of A and B as listed below: Efficacy of A efficacy of B Efficacy of A efficacy of B Efficacy of A = efficacy of B Efficacy of A efficacy of B Efficacy of A efficacy of B Quencher A was applied to the right axilla and B to the left, or vice versa. The quenchers were applied twice a day, once in the morning and once in the afternoon, for seven consecutive days during which the patients could bathe, but the usage of neither soaps nor deodorants was permitted. The axillae of the patients were evaluated by the assessors on the seventh day. The identity of A and B was kept blind to both the patient and the assessor, and only the supervisor who finally collected the results could distin- guish the two formulae. RESULTS AND DISCUSSION HEADSPACE GC ANALYSIS FOR EVALUATING QUENCHING ACTIVES If we keep in mind the quenching mechanism we are proposing here, the candidates under investigation should contain metallic elements, preferably with a mild alkaline effect, and needless to mention, must be safe on human skin. Several possible candi- dates to fulfill the above demands were analyzed by headspace GC. Isovaleric acid con- sumption values of the candidates are illustrated in Figure 4. Fine-particled zinc oxide was found to be most efficacious, followed by hydroxy apatite, known as a peptide adsorber. The most widely used antiperspirant ingredient, aluminum chlorhydrate, was superior compared to talc, which showed almost no effect at all, but was significantly ineffective in comparison with zinc oxide. The quenching mechanism of zinc oxide can be estimated as shown below: 2C4H9COOH q- ZnO--• (C4H9COO)2Zn q- H20 CONFIRMATION OF THE QUENCHING MECHANISM BY FT-IR The FT-IR spectrum of zinc oxide-treated short-chain fatty acid aqueous solution is shown in Figure 5. The strong absorption band observed near 1600 cm-1 can be as-
NOVEL POWDER DEODORANT 341 signed as the carboxylate anion of short-chain fatty acid zinc salt. The absence of an absorption at 1700 cm-•, which should be observed in the presence of free fatty acids, convinced us that the expected reaction as shown below was actually proceeding: 2RCOOH + ZnO-- (RCOO)2Zn q- H20 (R: alkyl group) 3000 .o_ 2000 (-) Figure 4. Isovaleric acid consumption values of various ingredients. The larger the value, the greater the efficacy of the ingredient to quench isovaleric acid odor.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)