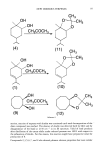
106 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 2.2 t.8 • 14 m ß o m 1 0 • ß Z - I. kl 0.6 0.2 TI02 TALC ZINC BLACK IRON OXIDE YELLOW R E D IRON OXIDE IRON OXIDE OXIDE __0.2 I I I I i I • I , I • ! I i I I. , I • I 300 400 500 600 700 WAVELENGTH (nm) Figure 1. Reflection spectra of physical sunscreen powders. The data plotted shows absorbance vs wave- length. An absorbance of 0.0 corresponds to 100% reflection of the light relative to BaSO4 an absorbance of 1.0 corresponds to 10% 2.0 corresponds to 1% reflection. be effective. If they do not appear visible as powders or as a film on the skin, they are not scattering light. It is tempting to speculate that a cosmetically acceptable product providing UV protection could be formulated by matching the index of refraction of barium sulfate in the visible with the index of refraction of the vehicle and having a large difference in index of refraction in the ultraviolet. However, this would be diffi- cult to accomplish, for when the indices of refraction between the scattering particles and the surrounding media are matched in the visible, the formula will disappear on the skin. At this point these particles no longer protect as a sunscreen because they are no longer scattering light. This means that the use of compounds like BaSO 4 and talc as physical sunscreens is limited because the scattering function is susceptible to alteration by the agent's environment. On the other hand, the use of titanium dioxide or zinc oxide in sunscreening products makes a great deal of sense. Both compounds exhibit a very strong absorption band at wavelengths just short of the visible spectrum. This corresponds to the optical band gap of these semiconductor-like materials. At wavelengths shorter than the optical gap, the radiation will excite electrons from the valence band to the conduction band. At wave- lengths longer than the optical gap, this mechanism for absorption and dissipation of radiant energy is not available. As the purity is compromised, the absorption edge becomes gradual and the band cut-off is less steep. This is seen in Figure 1, by com- paring titanium dioxide curves 1 and 2.
PHYSICAL SUNSCREENS 107 I..O z o i- z I..O 13:: o 400 500 600 WAVELENGTH {nm ) 700 Figure 2. Reflectance spectra of selected sunscreen powders using the fiber optic remittance spectrometer compared to BaSO 4 standard. a) Zinc oxide powder. b) Titanium dioxide powder. Our in vivo data for the ointment applied to skin shown in Figure 3 agree with the in vitro work with the ointment and with powders in Figure 1. The very sharp optical gap that begins just below 400 nm can be seen. At wavelengths shorter than the gap, the product should function as a very good broad-spectrum sunscreen. At wavelengths longer than 400 nm, protection will be provided by scattering. The in vitro results with powders suggest that high absorbance may be achieved on skin if properly formulated. The benefit of including the absorption of a physical sunscreen that has semiconductor- like properties is that the protection provided by the product will be unchanged by the surrounding media. Formulation techniques can be used to create aesthetically accept- able products using these optical properties. In sunscreen products where scattering is used as a means of attenuating radiation, the wavelength of the radiation will generally be shorter than the particle diameters of the material employed. This will result in the common Mie type of scattering, which varies relatively slowly with the wavelength of light. If particles are smaller than 0.03 microns in diameter, then Rayleigh scattering will take place. If scattering is to be used in sunscreen products to attenuate exposure, then the smaller- diameter particles will increase the concentration of particles on the skin's surface and attenuate more radiation for the same concentration of physical sunscreen. The particle radius cannot be indefinitely decreased, however. When the radius becomes substan- tially smaller than one micron, the scattered light will be preferentially in the forward direction, thereby deceasing the effectiveness of the material. Iron oxides are intended to provide color to the skin. As can be seen in Figure 1, the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)