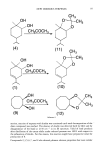
108 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS W Z rn 0 z w t.2 0.8 0.6 0.4 0.2 b 0 I ß I , I , I , I I I • I • I I 300 400 500 600 700 WAVELENGTH (nm) Figure 3. Rosa Cream contains 7.5% TiO2 and 7.5% ZnO. a) Opt{cally infinitely thick sample with quartz coverslip using remittance spectrometer. b) One mg/cm 2 on human skin using remittance spectrom- eter. Absorbance is twice as high as expected because radiation must pass twice through the sample before being analyzed. color is easily detected by measuring reflectance. The low reflectance extends through the ultraviolet. These compounds also should function as physical sunscreens because they absorb the potentially harmful wavelengths. CONCLUSIONS Users of physical sunscreen agents should recognize their common properties as particu- late powders insoluble in the formulation. In terms of their optical properties they 1) scatter visible and ultraviolet radiation equally well, or 2) scatter visible and absorb ultraviolet, or 3) scatter and absorb visible and ultraviolet to different extents. The scattering properties of these powders can be modified by matching their index of refraction with that of their vehicles, but the absorption properties are characteristic of the bulk material and cannot be modified. Reflection and scattering of light is effective as a means of protection only if the refrac- tive index of the medium used to disperse the physical sunscreen and that of the phys- ical sunscreen itself are different. The closer these indices are to one another, the lower the screening efficiency. Talc and barium sulfate, which are effective as sunscreen agents only by this mechanism, suffer from this problem. Iron oxides exhibit electronic absorption bands in the visible region, giving rise to their perceived color. These bands extend into the ultraviolet region, thereby resulting in a potential use as sunscreen agents. Because of the nature and degree of absorption in the
PHYSICAL SUNSCREENS 109 ultraviolet regions, these materials are not widely recognized for their sunscreen poten- tial, despite the fact that they should not suffer from the efficacy problems noted above for agents that only scatter and reflect such light. Titanium dioxide and zinc oxide each exhibit a strong semiconductor absorption in the ultraviolet. They also scatter and reflect only visible and ultraviolet light. Through careful formulation, aesthetically acceptable products can be made that minimize the scattering and reflection while simultaneously absorbing a significant amount of ultra- violet light because of the clear optical gap. The in vivo data presented herein confirm this point. The optical band gap exhibited in the formulated product resulting from the presence of titanium dioxide and zinc oxide can also be observed when product has been applied to the skin. In conclusion, users of physical sunscreen agents should recognize that there are impor- tant differences among these agents based upon their mechanism of solar light attenua- tion. The specific mechanism by which each agent attenuates radiation plays a crucial role in its applicability to particular formulation types. An understanding of the dif- ferent mechanisms of solar attenuation can potentially yield improved sunscreen efficacy through the combined use of physical and chemical sunscreen agents. ACKNOWLEDGMENTS We appreciate the use of the Varian Cary 2300 and Harrick Praying Mantis provided by Dr. M. Shah Jahan, Physics Department, Memphis State University, Memphis, TN 38152. REFERENCES (1) US FDA Monograph. Sunscreen products for over-the-counter human use: Proposed safety, effective and labeling conditions. Fed. Reg. 43: No. 166, Aug. 25, 1978, 38206-38269. (2) N. Kollias, A. Baqer, and K. Razi Naqvi, Fiber optic spectrophotometer for noninvasive transmission and diffuse reflection studies, Spectroscopy Letters, 19, 149-150 (1986). (3) M. A. Pathak, Sunscreens: Topical and systematic approaches for the preventior• of acute and chronic sun-induced skin reactions, Dermatol. Clin., 4, 321-33334, (1986).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)