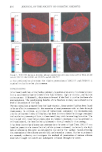
256 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS gradual absorbance of a vitamin E acetate droplet into a lameliar lecithin/water liquid crystalline phase during evaporation of water from an emulsion stabilized by lecithin. EXPERIMENTAL MATERIALS © Lecithin: Lec (extracted from egg), Kabi Chemical Co., Stockholm, Sweden © Vitamin E acetate: Tocopherol acetate, (+)-d-(T3001), Sigma Chemical Co., St. Louis MO © Water: H20, doubly distilled © Sudan 4: 85%, Sigma Chemical Co., St. Louis, MO PHASE DIAGRAM The maximum solubilization of vitamin E acetate into a lecithin/water lamellar liquid crystal was established by direct titration using water for different lecithin/vitamin E acetate ratios. The liquid crystalline phase was identified from its pattern in an optical microscope between crossed polarizers, and its phase limits were determined by low- angle x-ray diffraction. EVAPORATION The evaporation of the volatile component of the emulsion composed of 3% vitamin E acetate, 17% Lec, and 80% H20 was monitored using optical microscopy, and pho- tographs were taken in order to observe the interfacial transfer of vitamin E acetate into the lameliar liquid crystal. The vitamin E acetate was stained with Sudan 4 for improved contrast. LOW-ANGLE X-RAY DIFFRACTION The samples were introduced into special thin-g?ss capillary tubes of 0.5-mm diameter. A Ni-filtered Cu radiation was used (a-1.542 A) for the x-ray measurements, and the detector was a Tennelec gas ionization model (PSD-1100). The LAXD was used in the determination of the three-phase region as well. RESULTS Part of the phase diagram of the system water (H20), lecithin (Lec), and vitamin E acetate is shown in Figure 1. In the areas of interest one finds two liquid phases and one lameliar liquid crystal. The diagram is similar to earlier reports of such systems (10-12). The two liquids, water and vitamin E acetate, showed no significant solubility in each other, nor did they dissolve lecithin. A lamellar liquid crystalline phase was observed in the range of 64 to 97 percent by weight of lecithin on the water/lecithin axis. It solubilized a maximum of 13.5% by
TRANSFER OF VITAMIN E ACETATE 257 Vitamin E H•O ]..•c Figure 1. Ternary phase diagram showing the LLC (lameliar liquid crystal) and the three-phase emulsion region. x = 3% vitamin E/17% LEC/80% H20. weight of vitamin E acetate. The phases at higher lecithin content (13) are not of interest for the present investigation and were not examined. With these results, the phase diagram, Figure 1, consists of a three-phase region of water (H20), vitamin E acetate (vitamin E), and the lameliar liquid crystal (LLC) of water/lecithin in a 36/64 weight ratio. It should be observed that the liquid crystal in this three-phase equilibrium contains only insignificant amounts of vitamin E acetate. This three-phase region borders the higher lecithin content two-phase area in which vitamin E acetate is in equilibrium with the lameliar liquid crystalline phase. A phase diagram of this kind gives rise to some unexpected features during evaporation of an emulsion. In the present case, the composition trajectory during evaporation is a straight line, the backward extrapolation of which reaches the water corner because water is the only component with a significant vapor pressure to evaporate at room temperature. Hence, the composition during evaporation of an emulsion with initial composition A (Figure 1) follows the line A-F in the figure, and the following predic- tions about the change in appearance are obtained from the diagram: At the beginning, when water has the largest volume of three phases, 70.5% by weight at point A, the emulsion should be water-continuous with dispersed liquid crystal lumps (26.5%) as well as vitamin E acetate droplets (3%). With evaporation the amount of water is reduced, and at B the composition is 40% water combined with 54.3% liquid crystal and 6.1% vitamin E acetate. At point C the aqueous phase has disappeared completely only two phases remain in the emulsion: vitamin E droplets (11.3%) dispersed in the liquid crystal. Continued evaporation C-D (Figure 1) causes the droplets of vitamin E acetate to disappear, and only a liquid crystal now remains for the range D-F. At F, vitamin E acetate should again form separate droplets. In general, the straight-line trajectory should not be accepted based on the relative vapor pressures only. Recent contributions (14) have demonstrated beyond doubt that the evaporation path may be strongly bent at low water content, but these findings were concerned with lower water content and less difference in vapor pressure between water and organic solvent than is the case in the present investigation.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)