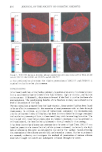
258 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS These predictions on phase changes were based on equilibrium conditions, and it was considered instructive to investigate the changes under conditions similar to those experienced after application of a skin lotion in a thin film. At first Figure 1 shows the water/liquid crystal ratio to change from 4.7 to 0 in the composition range A to C. Such a process of necessity means a reversal of the emulsion structure from water-continuous to liquid crystal-continuous. Figure 2 illustrates this reversal. The suspension of liquid crystals in water are a distinct feature of Figure 2A. The liquid crystal droplets gradually become the continuous phase in Figures 2B to 3D. The absorption of vitamin E acetate droplets during water evaporation is illustrated in Figure 3. The increase of the droplet through gradual dissolution of the vitamin E acetate into the liquid crystal is apparent from the photographs, and the entire disap- pearance of the droplet between G and H is obvious. The black spot in the center of the droplet is the organic dye that was used to distinguish the vitamin E acetate droplets from water droplets in the later phase of evaporation after inversion had take place. Continued evaporation, according to the phase diagram, would lead to a reappearance of the vitamin E acetate droplets at point F, Figure 1. Optical microscopy observation showed no macroscopic droplets to appear during the continued evaporation after point D. DISCUSSION The results, in general, illustrate the value of phase diagrams in the evaluation of the A B c D Figure 2. Evaporation of water gradually changes the emulsion from being water-continuous to liquid crystal-continuous, A-- D.
TRANSFER OF VITAMIN E ACETATE 259 A B E Figure 3. Evaporation of the small amount of water, C-D, in Figure 1 causes the droplet of vitamin E acetate to disappear into the liquid crystal, A-H. behavior of a skin lotion during evaporation. At first the inversion from a water- continuous emulsion (vitamin E acetate + lameliar liquid crystal)-in-water to a lameliar liquid crystal-continuous emulsion (water + vitamin E acetate)-in-lamellar liquid crys- tal was found experimentally (Figure 3). From a cosmetic point of view, this means that the patches of liquid crystal and vitamin E acetate in water that are found after appli- cation to the skin are replaced by a continuous layer of liquid crystal with droplets of water and vitamin E acetate in it. Continued evaporation causes the water droplets to disappear at point C (Figure 2), and now the remaining emulsion is a vitamin E acetate in lameliar liquid crystal emulsion. With continued evaporation, the droplets of vitamin E acetate disappear into the liquid crystal as confirmed by Figure 3. The vitamin E acetate is now homogenously distributed in the layer of lameliar liquid crystal. However, from a cosmetic point of view, even more essential information from Figure 1 is found in the conditions after continued evaporation to less water than at point D, Figure 1. The solubilization capacity of the lameliar liquid crystal for vitamin E acetate is now reduced, and droplets of it should appear in the system. No droplets were observed experimentally, which means that nucleation does not take place.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)