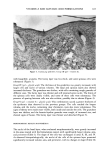
POLYETHYLENE GLYCOL-8/SMDI COPOLYMER 143 0.010 0.00g 0.006 0.004 0.002 0.000 ,[[ , [ i -2 0 2 4 6 $ 10 12 14 Percent polymer added (w/w) Figure 6. Effect of PP-15 on the apparent solubility of salicylic acid at 4øC. o 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0 -0.1 1.00e-4 1.00e-3 1.00e- 1 1.00e+0 Drug concentration 0Violes/lite0 Figure 7. PP-15 binding to salicylic acid and lactic acid. [•, Salicylic acid ß lactic acid. surface is desirable, the target tissue for SA is assumed to be the SC it is therefore advantageous to maintain significant SC levels for an extended period of time. However, SA is rapidly transported through the skin from simple solutions and suspensions (Figures 1,3), allowing most of the applied dose to permeate the skin within an eight hour period. Levels within both the SC and the viable tissues reach a peak at an early
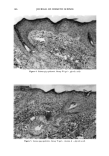
144 JOURNAL OF COSMETIC SCIENCE 50 45 40 35 30 25 20 15 10 5 0 0 0.1 0.2 4 6 8 10 12 Percent drug (w/w) Figure 8. Effect of drug concentration on the cloud point of PP-15. [•, Salicylic acid •, lactic acid. time (within the first two hours after application) and decrease thereafter (Figure 2), mirroring the rapid accumulation into the receptor. PP-15 changes the permeation kinetics of SA with 3% PP-15 in the formulation, the rate at which SA reaches the receptor is significantly reduced (Figures 1,4). A concomitant effect is an increase in SA concentrations within the SC during the first six hours after application. As will be discussed below, both of these differences result from binding of SA by PP-15. Because of binding, the thermodynamic activity of SA is reduced in the presence of PP-15, and the SC/vehicle partition coefficient is reduced. As a consequence, the transfer rate into SC is lower than for a control system, which also results in a lower rate of passage through the skin. SA concentrations persist in the donor, which continues to feed the SC, thus accounting for the maintenance of the level in this tissue for a longer time than is found for the control. Pretreatment of the skin with PP-15 does not affect the permeation rate of tritiated water (9). Furthermore, if silicone sheeting is substituted for skin in the diffusion cell, the same result is obtained, i.e., a reduction in SA penetration into the receptor (9). Not only that, but the relative reduction in penetration rate is essentially the same for both membranes. It is thus apparent that the effect of PP-15 on SA permeation is not related to any change in properties of the skin due to this polymer. The reduction in permeation is therefore dependent on an interaction occurring between the polymer and the active ingredient within the donor, or, in other words, a change in the thermodynamic activity of SA. The mechanism of interaction between PP-15 and SA was explored through measure- ment of the PP-15 cloud point, apparent solubility of SA, and SA binding by PP-15 (via equilibrium dialysis). The cloud point is a familiar property of many nonionic surfactants and polymers, particularly those based on a polyoxythelene chain (as is the case with
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)