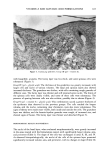
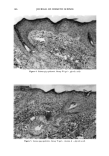
172 JOURNAL OF COSMETIC SCIENCE corneum results in dry skin and the formation of wrinkles. Therefore, it is useful to apply a humectant to skin to maintain the normal water content (12,13). As a humectant, sodium hyaluronate (HA) is a biopolymer derived from cartilage, vitreous humor, and synovial fluid, and shows useful physical characteristics such as high viscosity, viscoelasticity, high thread-forming ability, and high biocompatibility (14). For example, HA is used as a thickener in aqueous solvent systems, as a coating enforcement for liposomes, as an embedded base for organisms, capsule bases, and so on. On the other hand, many kinds of HA derivatives were synthesized and evaluated for useful functions. Abatangelo et •/. reported that sulfated hyaluronic acid derivatives showed no cytotoxic effects on mouse fibroblasts and that the introduction of sulfate groups along the hyaluronic acid chain made the macromolecules resistant to enzymatic digestion (15). In our study, to endow HA with precious functions, we synthesized a variety of HA derivatives and evaluated their usefulness for cosmetic products. After numerous investigations, we eventually discovered a novel HA derivative, sodium acetylhyaluronate (AcHA), which increases HA's moisturizing effect and has a very high skin-softening effect on stratum comeurn. In this study, we report the synthesis of AcHA that could show excellent moisturizing and skin-softening effects. We also report an i, vivo treatment effect of AcHA, which was used as an active ingredient in a lotion for dry skin condition. EXPERIMENTAL MATERIALS Chemicals used in this study were cosmetic grade or reagent grade without further purification. One 6-week-old male Hartley guinea pig (Japan SLC, Shizuoka, Japan) was housed individually in a wire mesh cage (260 x 380 x 180 mm, Japan Clea, Tokyo) and kept under standard laboratory animal conditions, which were maintained at a tem- perature of 21øC to 25øC with 40% to 70% relative humidity (RH). The room air was ventilated l0 to 15 times per hour automatically and a 12 hr/12 hr light-dark cycle (lighting 07:00-19:00) was imposed. The animal was fed •a•/ib•t•m with a commercial diet (RC-4, Oriental Yeast Industry, Tokyo) and sterilized water through an automatic water supply system. The animal was deeply anesthetized with pentobarbital sodium and the skin was dissected out. Animal care and experiments were performed in accor- dance with the guidelines of the National Institute of Health. The stratum corneum was removed from the dorsal skin by the heat-trypsinization method described by Chris- tophers and Kligman (16,17). The sheets of the stratum corneum were stored in a vacuum desiccator over silica gel. The water used in this study was purified by an ion-exchanged water purification system Type G-10B (Organ0, Tokyo, Japan). SYNTHESIS OF AcHA In a 300-ml conical glass flask, a mixture (100 ml) of acetic acid and acetic anhydride (1:4 - 4:1 by volume) was placed, and 6 g of fine powder of Biohyalo-12 © (sodium hyaluronate molecular weight: approx. 1,200 kilodaltons Shiseido, Tokyo) was gradu- ally added with stirring. Then, 3 - 5 ml of concentrated sulfuric acid as a catalyst was
EFFECT OF AcHA ON STRATUM CORNEUM 173 added dropwise to the stirred mixture for 10 - 360 min at room temperature (RT) (18). The reaction solution became viscous and clouded, and formed a fiber-like precipitate upon the addition of 2000 ml of water. The precipitate of acetylhyaluronate was col- lected by a cheesecloth and was washed twice with 2000 ml of water. The precipitate was dispersed into 250 ml of 80 v/v% aqueous acetone. Upon the addition of 50 w/w% sodium lactate (9 g), the precipitate dissolved completely with gentle stirring. When 400 ml of acetone was gradually added to the solution, the gel-like precipitate of sodium acetylhyaluronate (AcHA) appeared in the beaker. After being collected by decantation and washed twice with 100 ml ethanol by using a homogenizer (10,000 rpm) for 10 min, the precipitate was collected by filtration with reduced pressure and dried in a vacuum, and a white powder of AcHA was obtained. Acetyl group analysis showed that AcHA contained 2.6 - 3.8 acetyl groups per repeating disaccharide unit (namely, degree of substitution, DS), and static laser scattering measurement showed that the weight- average molecular weight of AcHA was approximately 150 kilodaltons. FT-IR analysis strongly supported the following structure of AcHA (Figure 1): [IR 3440 (-OH or -NH), 1740 (-C=O-), 1620 (-C=O-), 1375 (-CH3), 1250 (-C-O-), and 1050 cm -• (-C-O-C-)]. This polymer could dissolve in 90 w/w% aqueous ethanol. Since the surface tension of 1.0 w/w% AcHA was approximately 55.0 mN'm -•, which was lower than that of 1.0 w/w% HA (74.0 mN-m-•), AcHA shows the surface tension reducing ability. MEASUREMENT OF DYNAMIC ELASTIC MODULUS OF STRATUM CORNEUM The skin-softening effects of AcHA and conventional humectants were measured using the method reported by Takahashi eta/. (19). A stratum corneum strip (20 x 3 mm) was held horizontally by two clamps. The left clamp applied a fixed-amplitude sinusoidal stress (5 pm amplitude, 30 Hz) to the left end of the sample. The right clamp had a high-sensitivity sensor that detected the weak stress that was transmitted through the sample. The detected stress signal was calculated and transformed into a series of digital COONa I CH3COHN ' H _ o OR _ n R: H or CH3CO Figure 1. Structure of sodium acetylhyaluronate (AcHA).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)