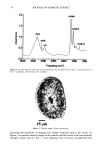
j. Cosmet. Sci., 51, 91-101 (March/April 2000) Structure-function relationships of dimethicone copolyol NELSON E. PRIETO and ANTHONY J. O'LENICK, Petroj•rm Inc., Fernandina Beach, FL (N.E.P.), and Siltech USA, Dacula, GA (A.J. O'L.). Accepted for publication January 31, 2000. INTRODUCTION Throughout the 1990s dimethicone copolyol (DMC) surfactants and their derivatives have been an important and growing class of surface-active agents. These materials are used in a diverse area of applications such as cosmetics, textiles, coatings, lubricants, and detergents, due to their ability to provide maximum surface-active properties in a cost-effective manner (1-3). Despite the growing use of these silicone-based compounds, studies regarding the basic understanding of the effect of their structure on surfactant properties remain limited, relative to the information openly available for organic sur- factants. The present study was undertaken to determine the effect of DMC structure on its surfactant properties. Properties studied included wetting, cloud point, critical micelie concentration (cmc), foaming, solubility, spreading, emulsification, and ocular irritation. The relationship of the results to application is discussed. DIMETHICONE COPOLYOL CHEMISTRY The term dimethicone copolyol has been adopted by the Cosmetics, Toiletry and Fra- grance Association to describe a class of silicone/polyoxyalkylene derivatives. DMC surfactants are a class of compounds that conform to the following general structure: CH 3 CH 3 CH 3 CH 3 CH3-Si--(-O--Si--)•--(O--Si)b--O--Si--CH 3 CH 3 CH 3 (CH2) 3 CH 3 I O-(CH2CH20)x-(CH2CH(CH3)O)yH The nomenclature was developed to reflect the fact that the molecule is (a) a silicone polymer (dimethicone), (b) a copolymer ("copoly" part), and (c) hydroxyl functional ("o1" 91
92 JOURNAL OF COSMETIC SCIENCE ending). The original concept, while creative, does not give the total information needed for defining the molecular structure. For example, the current practice is to call me- thoxy-capped products DMC, even though they lack the hydroxyl group that originally justified the "o1" ending. Since the capping process is not totally efficient, there are residual hydroxyl groups found even in the DMC compounds that claim to be capped. TERMINAL GROUP FUNCTIONALITY The structure of the DMC having a terminal group is shown below. For hydroxy- terminated DMC the structure is: CH 3 CH 3 CH 3 CH 3 CH3-Si--(-O--Si--)•-- ( O--Si)b--O--Si--CH 3 CH 3 CH 3 (CH2) 3 CH 3 I O-(CH2CH20)x-(CH2CH(CH3)O)yH For methoxy-terminated DMC the structure is: CH 3 CH 3 CH 3 CH 3 CH 3-Si--(-O--Si--)•--( O--Si)b--O--Si--CH 3 CH 3 CH 3 (CH2) 3 CH 3 I O-(CH2CH20)x-(CH2CH(CH3)O)yCH 3 The molecular structure is complicated further by the fact that the a, b, x, and y values vary quite considerably within the class and are generally not revealed by manufacturers. The raw materials used to synthesize these polymers are themselves polydisperse poly- mers having an oligomeric distribution. The resulting polymer is a complex oligomeric distribution of the initial oligomeric distribution. Despite the complications, which are not too unlike those found in ethoxylated fatty surfactants, silicone polymers can be analyzed and structure/function properties determined. The process used for their syn- thesis is reproducible and gives products with little variation, albeit complex mixtures (1•). The general reaction scheme for the synthesis of DMCs is as follows: CH 3 CH 3 CH 3 CH 3 CH3---Si---(-O---Si---)•---(-O--Si---)b--O--Si--CH3 CH 3 H CH 3 CH 3 + "a" moles of CH2=CH-CH2-O-(CH2CH20)x-H
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)